��Ŀ����
����Ŀ�����������һ����Ҫ�Ļ���ԭ�ϣ�����ɫ���Ʊ�����Ҫ�Ŀ��⡣ij����С��������·�����������Ʊ�������ء�
I. ������Ʊ������������̡�����غ��������ع����������������������ء�
��1������ʱ��___________(����������������������)��
��2����Ҫ�Ƶ������59.1g��������Ҫ�����___________g��
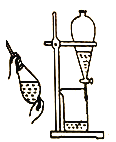
II. ��������Ʊ����Ʊ�������ص�װ������ͼ��ʾ(������г�װ��ʡ��)��
��I�Ƶõ�����ؼ���������ˮ�ܽ⣬��ī��ɫ��Һ����������ƿ�С���װ�÷�Һ©����������ϡ���ᣬ���ȣ���������������ͨ����װ�ã�ˮԡ���ȣ����裬�����������������Ӧ���ɸ�����غͶ������̡�

��3�������ӷ���ʽ��ʾ��װ���д��ڵĻ�ѧƽ��___________(��дһ��)��
��4����װ���з�����Ӧ�Ļ�ѧ����ʽ��______________________��
��5����װ��������������Һ��������______________________��
��6����װ���������������Ľ��Ĵ�ʩ��______________________��
��7���ж���װ�����������ȫ��Ӧ��ʵ�鷽���ǣ��ò�����պȡ��Һ�ε���ֽ�ϣ����۲쵽______________________�� ��ʾ���������ȫ��Ӧ��
III.������ز�Ʒ�Ĵ��ȷ�����
��8��������������Һ���ˣ�����Һ�����������У�Ũ������ȴ�����ˣ�ϴ�ӣ������KMnO4��Ʒ�������ʵ��֤����Ʒ�к�������MnO2��______________________��
���𰸡����� 12.25 H2O![]() H++OH�� 3K2MnO4+4CH3COOH =2KMnO4+ MnO2��+ 4CH3COOK+2H2O β�����������գ� �ڼ����м����Ӱ�ȫƿ �����Ϻ�ɫ��û��ī��ɫ�ۼ� ȡ������Ʒ����ˮ�����ˣ�ϴ�ӣ������ù�������Թ��У���������H2O2�������������ݣ���֤����Ʒ�к���MnO2
H++OH�� 3K2MnO4+4CH3COOH =2KMnO4+ MnO2��+ 4CH3COOK+2H2O β�����������գ� �ڼ����м����Ӱ�ȫƿ �����Ϻ�ɫ��û��ī��ɫ�ۼ� ȡ������Ʒ����ˮ�����ˣ�ϴ�ӣ������ù�������Թ��У���������H2O2�������������ݣ���֤����Ʒ�к���MnO2
��������
(1) ����״̬�������������벣���еĶ������跴Ӧ���ʽ���ʱ�������������ò���������Ϊ������
(2) ���������̡�����غ��������ع����������������������أ����������ԭ��Ӧԭ����ƽ�÷�Ӧ3MnO2+KClO3+6KOH=3K2MnO4+KCl+3H2O�����ݷ�Ӧ��֪����Ҫ�Ƶ������59.1g��������Ҫ�����![]() g��
g��
(3)��װ����ˮ�ĵ���ʹ���ĵ����Ϊ������̣������ӷ���ʽ��ʾΪH2O![]() H++OH����CH3COOH
H++OH����CH3COOH ![]() CH3COO-+H+��
CH3COO-+H+��
(4)��װ�������������ᷴӦ���ɸ�����ء��������̡�����غ�ˮ��������Ӧ�Ļ�ѧ����ʽΪ��3K2MnO4+4CH3COOH =2KMnO4+ MnO2��+ 4CH3COOK+2H2O��
(5)��װ��������������Һ��������β�����������գ���
(6)��װ���������������Ľ��Ĵ�ʩ���ڼ����м����Ӱ�ȫƿ��
(7)�ж���װ�����������ȫ��Ӧ��ʵ�鷽���ǣ��ò�����պȡ��Һ�ε���ֽ�ϣ����۲쵽�����Ϻ�ɫ��û��ī��ɫ�ۼ��� ��ʾ���������ȫ��Ӧ��
(8)ȡ������Ʒ����ˮ�����ˣ�ϴ�ӣ������ù�������Թ��У���������H2O2�������������ݣ���֤����Ʒ�к���MnO2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��(1)�����������Ƚ������ⷽ������������������������������Ӧϵͳͬʱͨ����顢������ˮ��������������Ҫ��ѧ��Ӧ�У�
��Ӧ���� | ��ѧ����ʽ | �ʱ���H(kJ��mol��1) |
���� ���� | CH4(g)+2O2(g)=CO2(g)+2H2O(g) | ��H1 |
CH4(g)+O2(g)=CO2(g)+2H2(g) | -322.0 | |
���� ���� | CH4(g)+H2O(g)=CO(g)+3H2(g) | +206.2 |
CH4(g)+2H2O(g)=CO2(g)+4H2(g) | +165.0 |
�ٷ�ӦCO(g)+H2O(g)=CO2(g)+H2(g)����H=________kJ��mol��1��
�ڼ����ȼ����Ϊ��H2������H2________��H1(����>������������<��)��
(2)������̼�ǵ�������ЧӦ��������ף�Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ���������ϳ�Ϊ�״����״�������ȼ�ϵ�ص���Ҫȼ�ϡ���֪�������״�ȼ�յ��Ȼ�ѧ����ʽ��
2H2(g)+O2(g)=2H2O(l)����H=-570 kJ��mol��1����
CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l)����H=-726.0 kJ��mol��1����
O2(g)=CO2(g)+2H2O(l)����H=-726.0 kJ��mol��1����
д��������̼�������ϳɼ״�Һ����Ȼ�ѧ����ʽ��___________��
(3)�пƼ�����������ϡ��������������Ϊ��������������˼״�����ȼ�ϵ�ء�����ϡ�������������ڸ������ܴ���O2-��
�������ص����������ķ�Ӧ��_______�����������ķ�Ӧ��________��
����ϡ��������Ĺ��������У�O2-���ƶ�������________��
����Ŀ�������й�ʵ��װ�á�����������ʵ����Ӧʵ��Ŀ�ĵ���
A | B | C | D | |
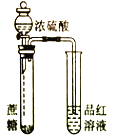
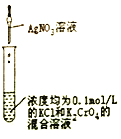
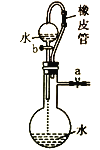
װ�� |
|
|
|
|
Ŀ�� | ��KOH��Һ��ȥ�屽�е����� | ֤��Ũ��������ˮ�ԡ�ǿ������ | �ȳ��ְ�ɫ�����������ש��ɫ������֤��Ksp(AgCl)< Ksp(Ag2CrO4) | ����ͨ���۲�ˮ�ܷ�ȫ���������ж�װ�������� |
A. A B. B C. C D. D