��Ŀ����
19���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһ��ѧԪ�أ�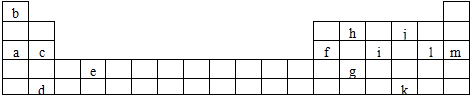
��1������Ԫ�صĵ��ʿ����ǵ�����������a c d e f h������ĸ���ţ���
��2���±���һЩ��̬��̬ԭ�ӵĵ�һ�����������ļ������ܣ�kJ•mol-1����
| � | X | Y | |
| ��һ������ | 519 | 502 | 580 |
| �ڶ������� | 7296 | 4570 | 1820 |
| ���������� | 11799 | 6920 | 2750 |
| ���ĵ����� | 9550 | 11600 |
�ڱ���Y����Ϊ����13��Ԫ���е�Al����Ԫ�ط��ţ�Ԫ�أ���Ԫ�ط��ű�ʾa��j�γɻ�����ĵ���ʽ��
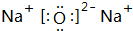 ��
�� ��
����3��д��Ԫ��e�ĵ����Ų�ʽ1s22s22p63s23p63d24s2��
��4������13��Ԫ���У�Ar����Ԫ�ط��ţ�Ԫ��ԭ��ʧȥ�����һ��������Ҫ��������࣮
��5������Ԫ��ԭ�ӵ���Χ�����Ų����������ɽ�Ԫ�����ڱ��ֳɼ����������У�Ԫ��c��m�ֱ�λ��s����p����
���� ��Ԫ�������ڱ���λ�ã���֪aΪNa��bΪH��cΪMg��dΪSr��eΪTi��fΪAl��gΪGe��hΪC��iΪP��jΪO��kΪTe��lΪCl��mΪAr��
��1�����������Ԫ���ǵ�������壬̼�ĵ���ʯīҲ���磬������ǽ������紦Ԫ�ؿ���Ϊ�뵼�壻
��2���ٸ��ݴﵽ�ȶ��ṹ����ʧȥ�������������ϸ߷�����
��Yԭ��ʧȥ������ĸ�����ʱ���������ҪԶԶ����ʧȥ�������������������������Y����㺬��3�����ӣ�ΪAlԪ�أ�
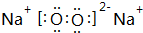
Na��O�γɵĻ�������Na2O��Na2O2��
��3��eΪ22��Ԫ��Ti�������������Ϊ22�����ݹ���ԭ��д�����������Ų�ʽ��
��4��ϡ������Ԫ�������ﵽ�ȶ��ṹ����ʧȥ��һ��������Ҫ�����ϴ�
��5�����������������ܼ����з�����
��� �⣺��Ԫ�������ڱ���λ�ã���֪aΪNa��bΪH��cΪMg��dΪSr��eΪTi��fΪAl��gΪGe��hΪC��iΪP��jΪO��kΪTe��lΪCl��mΪAr��
��1�����������Ԫ���ǵ�������壬̼�ĵ���ʯīҲ���磬Ge���ڽ�����ǽ������紦Ԫ��Ϊ�뵼�壬���и�Ԫ���У����������õ�����У�a c d e f h��
�ʴ�Ϊ��a c d e f h��
��2��������Liԭ��ʧȥ1�����Ӻ��γ��ȶ��ṹ����ʧȥ1�����Ӻ����ѣ������ԭ��ʧȥ����ڶ�������ʱ���������ҪԶԶ����ʧȥ��һ�����������������
�ʴ�Ϊ��Liԭ��ʧȥ1�����Ӻ��γ��ȶ��ṹ����ʧȥ1�����Ӻ����ѣ�
��Yԭ��ʧȥ������ĸ�����ʱ���������ҪԶԶ����ʧȥ�������������������������Y����㺬��3�����ӣ�ΪAlԪ�أ�
Na��O�γɵĻ�������Na2O��Na2O2�������ʽ�ֱ�Ϊ��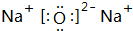 ��
�� ��
��
�ʴ�Ϊ��Al��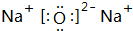 ��
�� ��
��
��3��Ti��ԭ������Ϊ22�����̬ԭ�ӵĺ�������Ų�Ϊ��1s22s22p63s23p63d24s2��
�ʴ�Ϊ��1s22s22p63s23p63d24s2��
��4������Ԫ���У�ArΪϡ�����壬�����Ϊ8�����ȶ��ṹ����ʧȥ��һ����������������ʴ�Ϊ��Ar��
��5��Ԫ��cΪMg������������s�ܼ����������ڱ���s����mΪAr������������p�ܼ����������ڱ���p�����ʴ�Ϊ��s��p��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰Ԫ�����ڱ�����������Ų��������ܵȣ�ע���������������������ϵ��

| A�� | 129C60���NA������ | |
| B�� | 46g NO2��N2O4�Ļ�����庬�е�ԭ����Ϊ3NA | |
| C�� | ��״���£�22.4L CCl4�����ķ�����Ϊ6.02��1023 | |
| D�� | 100mL0.1mol/LCaCl2��Һ��Cl-�����ʵ���Ũ��Ϊ0.01mol/L |
| A�� | ��SO2ͨ�����Ը��������Һ�У���Һ��ɫ������˵��SO2����Ư���� | |
| B�� | Ũ�����Ũ���ᱩ¶�ڿ�����Ũ�ȶ��ή�ͣ���ԭ����ͬ | |
| C�� | ��ij��ɫ��Һ�еμ��Ȼ�����Һ��������ɫ�������ټ���ϡ�����ó������ܽ⣬˵��ԭ��Һ��һ������SO42- | |
| D�� | �����е���п��ZnS����������ͭ��Һת��Ϊͭ����CuS����˵��CuS���ȶ��������л�ԭ�� |
��Ӧ��CO��g��+2H2��g��?CH3OH��g����H1
��Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H2
�±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250�� | 300�� | 350�� |
| K | 2.0 | 0.27 | 0.012 |
c��CO��=0.4mol/L������ɵô˶�ʱ��ķ�Ӧ���ʣ���H2��ʾ��Ϊ0.24mol/��L•min����
��2���ɱ��������жϡ�H1��0 �����������������=������
��ӦCO2��g��+H2��g��?CO��g��+H2O��g����H3=��H2-��H1 ���á�H1�͡�H2��ʾ����
��3���������ݻ����䣬�����д�ʩ����߷�Ӧ����COת���ʵ���b ������ĸ��ţ���
a������CO��ʹ��ϵ��ѹǿ���� b����CH3OH��g������ϵ�з���
c������He��ʹ��ϵ��ѹǿ���� d��ʹ�ø�Ч����
��4��д����Ӧ��Ļ�ѧƽ�ⳣ������ʽ��K��=$\frac{c��CH{\;}_{3}OH��•c��H{\;}_{2}O��}{c��CO{\;}_{2}��•c{\;}^{3}��H{\;}_{2}��}$�����ֺ��º��ݣ�����Ӧ���ƽ����ϵ�и�����Ũ�Ⱦ�����Ϊԭ����2������ѧƽ�������ƶ��������������������ƽ�ⳣ��K�����䣨��������С�����䡱����
| A�� | ������̼������ͨ���Ӿ۷�Ӧ�Ƶõ� | |
| B�� | ����ҵ�����������̼�������ϣ������ڻ�������ЧӦ | |
| C�� | ������̼���ϲ����ڿ�����ȼ�� | |
| D�� | �۶�����̼���ϵ�ʹ�û������ɫ��Ⱦ |
 H2SiO3��+2OH-������SiO32-+3H2O
H2SiO3��+2OH-������SiO32-+3H2O H4SiO4��+2OH-���������ӷ���ʽ��ʾ����
H4SiO4��+2OH-���������ӷ���ʽ��ʾ����
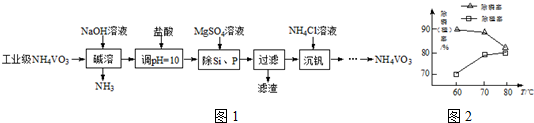
 ������ͼ�ش����⣺
������ͼ�ش����⣺ ��
��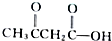 ����д��A��B�Ľṹ��ʽ��
����д��A��B�Ľṹ��ʽ��