��Ŀ����
10�������������зḻ�ĵ�Ԫ����Ҫ�Ե⻯�ص���ʽ���ڣ���ѧС�����ú�����ȡ�����ⵥ�ʣ��Ƚ������ճɻң�����ˮ����һ��ʱ�䣨�õ⻯�����ܽ⣩���õ�����������Һ��Ȼ���������̣�ͼ1����ȡ���ʵ⣨��֪��2I-+Cl2=2Cl-+I2������1������ ����ʹ�õIJ�������©�����ձ�����������
��2�����̢��г�������Cl2��Ŀ����ʹ������ת��Ϊ�ⵥ�ʣ�
��3����ȡ��Ĺ�����ʵ������۵����ƣ���ȡ������ȡ��Һ����ѡ����л��Լ�������BD������ţ��� A���ƾ� B�����Ȼ�̼ C������ D����
��4������ �ܴӺ�����л���Һ����ȡ�Ⲣ�����л��ܼ�������Ҫ����������������ͼ2��������ˮ������ΪB����A���A��B������B��A��������

���� ����ȡ���ʵ��ʵ�����̿�֪����Ϊ���ˣ���Һ�к������ӣ����з���Cl2+2I-=I2+2Cl-���õ���ˮ��Һ����Ϊ��ȡ��Һ�õ�������л���Һ����Ϊ���ɵõ���̬�⣮
��1������װ����������д�����ƣ�
��2�������������������������������ɵ��ʵ⣻
��3���ӵ�ˮ�л�ȡ�ⵥ�ʲ�����ȡ�ķ�������ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ԭ�ܼ��е��ܽ�ȣ����ʺ���ȡ������Ӧ����ȡ����ԭ���ܼ����ܻ��ܣ����Ȼ�̼������ȡ����ѡȡ�������Կ��������Ȼ�̼����ȡ��������������Һ���˵õ�����-����Һ��ͨ���������������ӵõ���ˮ��Һ���������Ȼ�̼������ȡ�ⵥ�ʣ�����õ��ⵥ�ʣ���4��ˮ����������Ч���ã�
��� �⣺��1����Ϊ���ˣ��õ����������ձ���©�����������ȣ��ʴ�Ϊ��©�����ձ�����������
�ʴ�Ϊ��©�����ձ�����������
��2��������������û������ӣ��䷴Ӧ���ӷ���ʽΪCl2+2I-=I2+2Cl-��ʹ������ת��Ϊ�ⵥ�ʣ�
�ʴ�Ϊ��ʹ������ת��Ϊ�ⵥ�ʣ�
��3���ӵ�ˮ�л�ȡ�ⵥ�ʲ�����ȡ�ķ���������������Һ���˵õ�����-����Һ��ͨ���������������ӵõ���ˮ��Һ���������Ȼ�̼������ȡ�ⵥ�ʣ�����õ��ⵥ�ʣ��ƾ��ʹ���������ˮ��������ȡ������ѡBD��
�ʴ�Ϊ����ȡ������ȡ��Һ����BD��
��4������ �ܴӺ�����л���Һ����ȡ�Ⲣ�����л��ܼ�������Ҫ����������������ͼ��������ˮ������ΪB����A��
�ʴ�Ϊ��B����A��
���� ���⿼���˺�ˮ��Դ���ۺ����ã��漰֪ʶ��϶࣬����ʵ�����̼������ķ�Ӧ����ȡ����ѡȡ��������ʵ��ԭ����װ�õ�֪ʶ��Ϊ���Ĺؼ������ظ�Ƶ����Ŀ��飬�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | H2O ��g���TH2��g��+$\frac{1}{2}$O2��g������H�T-485 kJ/mol | B�� | 2H2��g��+O2��g���T2H2O��g������H�T-485 kJ/mol | ||
| C�� | 2H2��g��+O2 ��g���T2H2O��g������H�T+485 kJ/mol | D�� | H2O ��g���TH2��g��+$\frac{1}{2}$O2��g������H�T+485 kJ/mol |
��1��������0.1mol/L��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С����A��
A��c��H+�� B��c��H+��/c��CH3COOH��
C��c��H+��•c��OH-�� D��c��OH-��/c��H+��
��2��ȡ10mL������Һ������������ˮ������ĵ���ƽ�����ң�����������ҡ��������ƶ�����ȡ10mL������Һ������������ˮ�����ƹ��壨����������ǰ����Һ������ֲ��䣩���������ܽ����Һ��c��H+��/c��CH3COOH���ı�ֵ����С�����������С������ȷ��������
��3����ͬ�����£�ȡ������ļס�������Һ����ϡ��100����ϡ�ͺ����Һ����pH��С��ϵΪ��pH���ף���pH���ң��� ���������������=������
��4��ȡ������ļס�������Һ���ֱ��õ�Ũ�ȵ�NaOHϡ��Һ�кͣ������ĵ�NaOH��Һ�������С��ϵΪ��V���ף��� V���ң��� ���������������=������
��5����֪25��ʱ��������ĵ���ƽ�ⳣ�����£�
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ��K1 | 1.8��10-5 | 4.3��10-7 | 3.0��10-8 |
| K2 | -- | 5.6��10-11 | -- |
A��HCO3- B��CO32- C��ClO- D��CH3COO-��
| A�� | ����CO2�����ʯ��ˮ��Ӧ��Ca2++2OH-+CO2�TCaCO3��+H2O | |
| B�� | ��KHSO4��Һ�м���Ba��OH��2��Һ��������Һ��pH=7 Ba2++OH-+H++SO42-=BaSO4��+H2O | |
| C�� | ��Ca��HCO3��2��Һ�е��������NaOH��ҺCa2++2HCO3-+2OH-=CaCO3��+CO32-+2H2O | |
| D�� | ����ͭ��Һ������������Һ��Ӧ��Ba2++SO42-=BaSO4�� |
| A�� | ��AgBr����ˮ�в��ܵ��磬��AgBr���ǵ���� | |
| B�� | CO2����ˮ�õ�����Һ�ܵ��磬����CO2�ǵ���� | |
| C�� | ���ڵĽ����ܵ��磬���Խ����ǵ���� | |
| D�� | ��̬��NaCl�����磬�� NaCl�ǵ���� |
| ���� | ���� | �������Һ | |
| A | Fe | Cu | FeCl2��Һ |
| B | Cu | Fe | ϡH2SO4 |
| C | Cu | Fe | CuSO4��Һ |
| D | ʯī | Fe | CuCl2��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 0.24mol•L-1 | B�� | 0.25mol•L-1 | C�� | 0.34mol•L-1 | D�� | 0.35mol•L-1 |
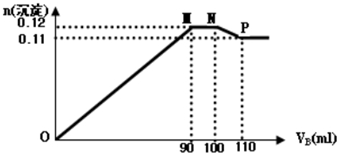
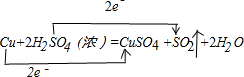 ���÷���ʽ��������ΪH2SO4����������Ԫ��ΪCu��
���÷���ʽ��������ΪH2SO4����������Ԫ��ΪCu��