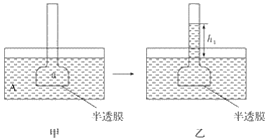
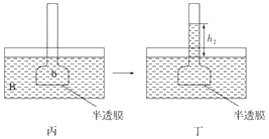
��Ŀ����
����Ŀ������ʽ�DZ����л�������ṹ��һ�ַ�ʽ����H2N-CH2-CH2-OH�ü���ʽ���Ա���Ϊ![]()
���ͪ(H)��һ������Ѫ���没��ҩ��ϳ�·������ͼ��ʾ��
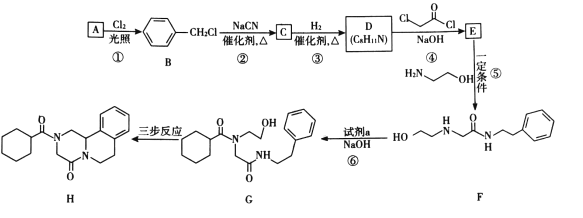
��֪i.R-Cl+NaCN![]() R-CN+NaCl
R-CN+NaCl
ii.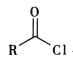 +R��-NH-R��+NaOH
+R��-NH-R��+NaOH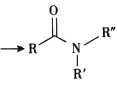 +NaCl+H2O
+NaCl+H2O
(1)A���ڷ���������������____��
(2)�ڵķ�Ӧ������____��
(3)B��һ��ͬ���칹�壬�������Һ˴Ź��������г�������壬��ṹ��ʽΪ____��
(4)����1 mol C��������Ҫ����____mol H2����D��
(5)�ܵĻ�ѧ����ʽ��____��
(6)F�����������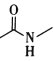 ��____��
��____��
(7)�Լ�a�Ľṹ��ʽ��____��
(8)G��H��Ϊ������Ӧ��J�к���3����Ԫ����д��I��J�Ľṹ��ʽ______��______��

���𰸡��ױ� ȡ����Ӧ ![]() 2
2  �ǻ�����-OH��
�ǻ�����-OH��  I��
I�� J��
J��
��������
BΪ![]() ��A��Cl2�ڹ��������·�������ϵ�ȡ����Ӧ����B����AΪ
��A��Cl2�ڹ��������·�������ϵ�ȡ����Ӧ����B����AΪ![]() ��B������֪i����Ӧ���ɵ�CΪ
��B������֪i����Ӧ���ɵ�CΪ![]() ��C��H2�ڴ������������¼��ԭ���ɵ�DΪ
��C��H2�ڴ������������¼��ԭ���ɵ�DΪ![]() ��D��
��D��![]() ������֪ii�ķ�Ӧ����EΪ
������֪ii�ķ�Ӧ����EΪ![]() ��E��һ�������º�
��E��һ�������º�![]() ��Ӧ����F��F���Լ�a��NaOH�����·���ȡ����Ӧ����G���Ա�G��F�Ľṹ��֪�Լ�aΪ
��Ӧ����F��F���Լ�a��NaOH�����·���ȡ����Ӧ����G���Ա�G��F�Ľṹ��֪�Լ�aΪ![]() ��G��������Ӧ�õ�F���ݴ˽��
��G��������Ӧ�õ�F���ݴ˽��
(1)�������Ϸ�����AΪ![]() ���������Ǽױ���
���������Ǽױ���
�ʴ�Ϊ���ױ���
(2) B������֪i�ķ�Ӧ���ɵ�CΪ![]() ����ԭ�ӱ�-CNȡ������Ӧ������ȡ����Ӧ��
����ԭ�ӱ�-CNȡ������Ӧ������ȡ����Ӧ��
�ʴ�Ϊ��ȡ����Ӧ��
(3)BΪ![]() ����ͬ���칹�壬�������Һ˴Ź��������г�������壬˵��������ԭ���ڱ������Ҵ��ڶ�λ����ṹ��ʽΪ
����ͬ���칹�壬�������Һ˴Ź��������г�������壬˵��������ԭ���ڱ������Ҵ��ڶ�λ����ṹ��ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
(4) C��H2�ڴ������������¼��ԭ���ɵ�DΪ![]() ��-C
��-C![]() Nת��Ϊ-CH2-NH2������1 mol C��������Ҫ����2mol H2����D��
Nת��Ϊ-CH2-NH2������1 mol C��������Ҫ����2mol H2����D��
�ʴ�Ϊ��2��
(5) D��![]() ������֪ii�ķ�Ӧ���ɵ�EΪ
������֪ii�ķ�Ӧ���ɵ�EΪ![]() ����ѧ����ʽΪ
����ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
(6)��F�Ľṹ![]() ��֪��������������
��֪�������������� ���ǻ�����-OH����
���ǻ�����-OH����
�ʴ�Ϊ���ǻ�����-OH����
(7) F���Լ�a��NaOH�����·���ȡ����Ӧ����G���Ա�G��F�Ľṹ��֪�Լ�aΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
(8)GΪ ��G��Cu/O2������ʱ��������������IΪ
��G��Cu/O2������ʱ��������������IΪ ��I����ȩ���ϵļӳɷ�Ӧ����JΪ
��I����ȩ���ϵļӳɷ�Ӧ����JΪ ��J�ɻ���ˮ����HΪ
��J�ɻ���ˮ����HΪ ��
��
�ʴ�Ϊ��I�� ��J��
��J�� ��
��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�����Ŀ������400 mL 0.5 mol��L��1��NaOH��Һ���Իش��������⣺
��1�����㣺��ҪNaOH���������Ϊ______��
��2��ijѧ����������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ���� ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�______(��������������������)�ұߵ����̡���ʹ��ƽƽ�⣬�����еIJ���Ϊ_______���ٶ����ճƵ�С�ձ�������Ϊ______(����32.6 g������31.61 g��)��
��3�����Ʒ������������������裺
�� ��ʢ��NaOH���ձ��м���200 mL����ˮʹ���ܽ⣬����ȴ�����£�
�� ����������ƿ�м�����ˮ��Һ��ӽ��̶���1��2 cm����
�� ��NaOH��Һ�ز�����ע��500 mL����ƿ�У�
�� ���ձ��м�������������ˮ��С��ϴ��2��3�κ���������ƿ��
�� ���ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȡ�
�Խ����ϲ����ų��Ⱥ�˳��______��
��4��ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48 mol��L��1��ԭ�������______��
A��ʹ����ֽ�����������ƹ��� |
B������ƿ��ԭ��������������ˮ |
C���ܽ�NaOH���ձ�δ�����ϴ�� |
D����ͷ�ιܼ�ˮ����ʱ���ӿ̶� |
��5������������0.5 mol��L��1NaOH��Һ����ʾ��ͼ���д������(�����)______��

����Ŀ��ij�¶���2 L�ܱ������У�3��������ʼ״̬��ƽ��״̬ʱ�����ʵ���(n)���±���ʾ��
X | Y | W | |
n(��ʼ״̬)/mol | 2 | 1 | 0 |
n(ƽ��״̬)/mol | 1 | 0.5 | 1.5 |
����˵����ȷ����(����)
A. �����¶ȣ���W�����������С����˷�Ӧ��H>0
B. ���¶��£��˷�Ӧ��ƽ�ⳣ��K��6.75
C. ����ѹǿ�������淴Ӧ���ʾ�����ƽ��������Ӧ�����ƶ�
D. ���¶��£�����������м���1.5 mol W���ﵽ��ƽ��ʱ��c(X)��0.25 mol��L��1