��Ŀ����
����Ŀ�������ܡ����ȵ��ʼ��������Ӧ�ù㷺��
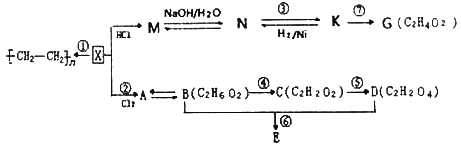
��1��LiFePO4������ӵ�ز��ϣ�����Fe2+�ļ۲�����Ų�ͼ____________������PO43-�����幹����____________��
��2���������̪ݼ���������������ε�صij�ŵ�Ч�ʣ���ͼ�Ǹ��ԡ�������̪ݼ�����ӵĽṹͼ��
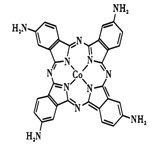
��д��������̪ݼ��N ԭ�ӵ��ӻ��������____________��
��д��һ���백��������NH2-��Ϊ�ȵ���������Է���____________��
�۽�����̪ݼ������Ϊ��������̪ݼ������ʹ��ˮ���Եõ���Ч���ƣ��������ԭ��____________
��3��1mol[Co(NO2)6]3-�������ĦҼ���Ŀ��____________��K3[Co(NO2)6]������Ԫ�صĵ�һ�������ɴ�С��˳����____________��
��ͼ��ʾΪNiO����ľ����ṹʾ��ͼ:
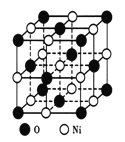
�ٸþ�����ռ����������������____________��
�ڼ�֪��NiO������Ni2+�İ뾶Ϊa pm��O2-�İ뾶Ϊbpm�������ھ������ǽ��ܽӴ��ģ�����NiO������ԭ�ӵĿռ�������Ϊ____________�����ú���ĸa��b�ļ���ʽ���
���𰸡� 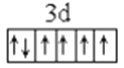 �������� sp2��sp3 H2O��H2S�� ����İ����ϵ�Hԭ�ӿ���ˮ�����γ���� 18NA N>O>Co>K 8
�������� sp2��sp3 H2O��H2S�� ����İ����ϵ�Hԭ�ӿ���ˮ�����γ���� 18NA N>O>Co>K 8 ![]() ��
��![]() ��100%
��100%
����������1������ԭ��������26����Fe2+�ļ۲�����Ų�ͼΪ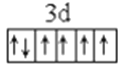 ������PO43-��P�ļ۲���Ӷ�����4���Ҳ����ڹ¶Ե��ӣ��������幹�����������塣��2���ٰ�����̪ݼ��N ԭ�Ӽ����γ�˫����Ҳ���γɵ��������ӻ��������Ϊsp2��sp3����ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬��������NH2-��Ϊ�ȵ���������Է���Ϊ H2O��H2S�ȣ��۽�����̪ݼ������Ϊ��������̪ݼ������������İ����ϵ�Hԭ�ӿ���ˮ�����γ������������ʹ��ˮ���Եõ���Ч���ƣ���3���������ǦҼ���˫���к���1���Ҽ�����1mol[Co(NO2)6]3-�������ĦҼ���Ŀ�� 18NA���ǽ�����Խǿ����һ������Խ��Ԫ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ����K3[Co(NO2)6]������Ԫ�صĵ�һ�������ɴ�С��˳����N>O>Co>K����4���ٸþ����������Ӹ�����8��1/8+6��1/2=4�������Ӹ�����12��1/4+1=4�����ռ����������������8���ھ����й�����4��Ni2+��4��O2�������Ϊ��
������PO43-��P�ļ۲���Ӷ�����4���Ҳ����ڹ¶Ե��ӣ��������幹�����������塣��2���ٰ�����̪ݼ��N ԭ�Ӽ����γ�˫����Ҳ���γɵ��������ӻ��������Ϊsp2��sp3����ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬��������NH2-��Ϊ�ȵ���������Է���Ϊ H2O��H2S�ȣ��۽�����̪ݼ������Ϊ��������̪ݼ������������İ����ϵ�Hԭ�ӿ���ˮ�����γ������������ʹ��ˮ���Եõ���Ч���ƣ���3���������ǦҼ���˫���к���1���Ҽ�����1mol[Co(NO2)6]3-�������ĦҼ���Ŀ�� 18NA���ǽ�����Խǿ����һ������Խ��Ԫ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ����K3[Co(NO2)6]������Ԫ�صĵ�һ�������ɴ�С��˳����N>O>Co>K����4���ٸþ����������Ӹ�����8��1/8+6��1/2=4�������Ӹ�����12��1/4+1=4�����ռ����������������8���ھ����й�����4��Ni2+��4��O2�������Ϊ��![]() �������ı߳�Ϊ2a+2b���������Ϊ��2a+2b��3���Ȼ��ƾ��������ӵĿռ�������Ϊ
�������ı߳�Ϊ2a+2b���������Ϊ��2a+2b��3���Ȼ��ƾ��������ӵĿռ�������Ϊ![]() ��100%
��100%