��Ŀ����
����Ŀ���л�������X������̼��������Ϊ6:1��E�Ľṹ��ʽΪ��![]() �������������ת����ϵͼ�ش����⣺
�������������ת����ϵͼ�ش����⣺
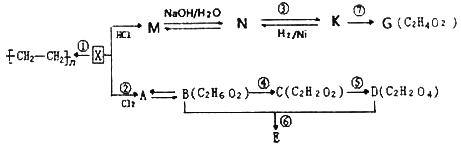
��1��K�й����ŵĽṹʽΪ��__________��C�Ļ�ѧ������____________��
��2��д�����з�Ӧ�ķ�Ӧ���ͣ�M��N____________����Ӧ��____________________��
��3��д������A�Ľṹ��ʽ��______________________________________________��
��4��������K��������Һ��Ӧ����ѧ����ʽΪ__________________________________��
��5����һ�����Ϳɼ���N��K��G�������ʣ��û�ѧ�Լ�Ϊ__________________________��
��6��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��_________________________��
��7����G��Ϊͬϵ��ұ�G��3��̼ԭ�ӵ����ʵ�ͬ���칹���������_______��
���𰸡� ![]() �Ҷ�ȩ ȡ����ˮ�ⷴӦ ������Ӧ CH2Cl-CH2Cl CH3CHO+2AgI[(NH3)2]OH
�Ҷ�ȩ ȡ����ˮ�ⷴӦ ������Ӧ CH2Cl-CH2Cl CH3CHO+2AgI[(NH3)2]OH![]() 2Ag��+CH3COONH4+H2O+3NH3�� ����Cu(OH)2��Һ HOH2C-CH2OH+HOOC-COOH
2Ag��+CH3COONH4+H2O+3NH3�� ����Cu(OH)2��Һ HOH2C-CH2OH+HOOC-COOH![]() +2H2O 4
+2H2O 4
��������X�����Ӿ۷�Ӧ���ɾ���ϩ���������̼��������Ϊ6:1����XΪCH2=CH2��MΪCH3CH2Cl��NΪCH3CH2OH��KΪCH3CHO��GΪCH3COOH��AΪCH2ClCH2Cl��BΪCH2OHCH2OH��CΪOHCCHO��DΪHOOCCOOH��
(1)CH3CHO�й����ŵĽṹʽΪ![]() ��OHCCHO�Ļ�ѧ�������Ҷ�ȩ��
��OHCCHO�Ļ�ѧ�������Ҷ�ȩ��
(2)M��N��Ӧ�ķ�Ӧ������±������ˮ�⣬��ȡ����Ӧ����Ӧ����ȩ����������������Ӧ��
(3)����A�Ľṹ��ʽΪCH2ClCH2Cl��
(4)CH3CHO��������Һ��Ӧ�Ļ�ѧ����ʽΪCH3CHO+2AgI[(NH3)2]OH![]() 2Ag��+CH3COONH4+H2O+3NH3����
2Ag��+CH3COONH4+H2O+3NH3����
(5)NΪCH3CH2OH��KΪCH3CHO��GΪCH3COOH����ѡ�����Ƶ�������ͭ�������������ܽ�������ͭ��Ϊ���ᣬ��������ש��ɫ������Ϊ��ȩ��
(6)�Ҷ������Ҷ�������������Ӧ�Ļ�ѧ����ʽΪ HOH2C-CH2OH+HOOC-COOH![]() +2H2O��
+2H2O��
(7)��CH3COOH��Ϊͬϵ��ұ�CH3COOH��3��̼ԭ�ӵ��л���Ϊ���ᣬ��4��ͬ���칹�壬�ֱ�ΪCH3CH2CH2CH2COOH��(CH3)2CHCH2COOH��CH3CH2CH(CH3)COOH��(CH3)3CCOOH��

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�����Ŀ���±���ʾʵ�飬����ͽ��۾���ȷ���ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ��Ũ�Ⱦ�Ϊ0.lmol��L-1NaCl��NaI�����Һ�еμ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp(AgCl)>Ksp(AgI) |
B | �����£��ⶨ�����ʵ���Ũ�ȵ�Na2CO3��Na2SO3��Һ��pHֵ | ǰ�ߵ� pHֵ�Ⱥ��ߵĴ� | �ǽ����ԣ�S>C |
C | ��ij��Һ�еμ��������� | ��Һ�г��������ݺ͵���ɫ���� | ��Һ�к���S2-�� SO32- |
D | ��FeCl3��KSCN�����Һ�У���������KCl�Ĺ��� | ��Һ��ɫ��dz | FeCl3 +KSCN |
A. A B. B C. C D. D