��Ŀ����
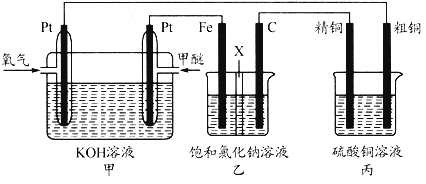
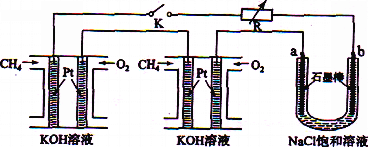
����Ŀ����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ��������������Ϊԭ������һϵ�л�����Ʒ��Ϊ���ԭ�ϵ������ʣ����ܽ��ġ������ͼ��ʾ�������̣������ȼҵװ���еĵ缫δ�����
(1)��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ___________________��
(2)Ϊ��ȥ�����е�Ca2+��Mg2+��SO42-����ɳ���õ�������NaCl���ɽ���������ˮ����ȷ�IJ��������˳����_______ (����ţ���
�ٹ��ˢڼӹ���NaOH��Һ�ۼ���������ܼӹ���Na2CO3��Һ�ݼӹ���BaCl2��Һ
A.�٢ܢ٢ڢݢ� B.�٢ڢݢܢ٢� C.�٢ڢܢݢ� D.�ܢڢ�
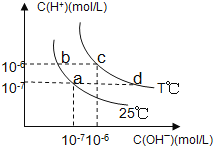
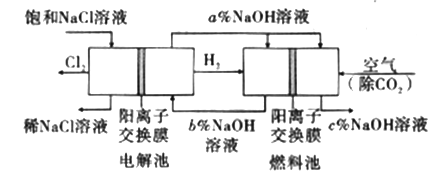
(3)ͼ��NaOH��Һ����������a%��b%��c%���ɴ�С��˳��Ϊ_________��
(4)�ȼҵ�IJ���NaOH�벻ͬ���ʷ�Ӧ�������ɲ�ͬ���Ρ���֪�����£�Ũ�Ⱦ�Ϊ0.1 mol/L������������ҺpH���±�������˵������ȷ����_______(�����)��
���� | Na2CO3 | NaHCO3 | NaClO | NaHSO3 |
pH | 11.6 | 9.7 | 10.3 | 5.2 |
A.����ˮ�м���NaHCO3������������ˮ�д������Ũ��
B.������Һ�У�ˮ�ĵ���̶�������NaClO
C.�����£���ͬ���ʵ���Ũ�ȵ�H2SO3��H2CO3��HClO��pH������HClO
D.�����ε������ӽ��H+������ǿ����HCO3-
(5)�����õ���Ȼ�����Һ���õ�������36.5%��Ũ����100t��������Ҫ����ʳ��_________t��
(6)�ȼҵ��ƷCl2������ұ��������õ������ѣ�������ͼ��д���������Ȼ����õ����Ȼ��ѵĻ�ѧ����ʽ��____________________��
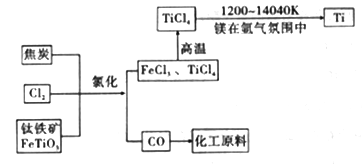
(7)��������������ֻ�ѧ��������ȶ������塣þ��ԭTiCl4�ķ�Ӧ��Ϊ��ֹMg��Ti������ѡ��ϡ��������������û�ѧ��Ӧ����ʽ���Ͳ�ѡ�õ�����ԭ��__________________��
���𰸡� 2NaCl��2H2O![]() 2NaOH��H2����Cl2�� B c%>a%>b% BD 58.5 2FeTiO3+6C+7Cl2=2FeCl3+2TiCl4+6CO 3Mg +N2
2NaOH��H2����Cl2�� B c%>a%>b% BD 58.5 2FeTiO3+6C+7Cl2=2FeCl3+2TiCl4+6CO 3Mg +N2![]() Mg3N2
Mg3N2
�����������⿼�黯ѧ�������̣���1����ⱥ��ʳ��ˮ�Ļ�ѧ��Ӧ����ʽΪ2NaCl��2H2O![]() 2NaOH��H2����Cl2������2����ȥCa2����Na2CO3����ȥMg2����NaOH����ȥSO42����BaCl2�����Ӳ������������ʣ���̼���ƻ��г�ȥ������BaCl2�����ã���Na2CO3����BaCl2�ĺ��棬���˳����NaOH��BaCl2��Na2CO3����BaCl2��NaOH��Na2CO3����BaCl2��Na2CO3��NaOH��Ȼ����ˣ��ټ������ᣬ��ѡ��B��ȷ����3������������һ�����缫��ӦʽΪ2H2O��2e��=H2����2OH�������a%>b%��ͨ����һ������O2��2H2O��4e��=4OH���������c%>a%,�����c%>a%>b%����4����������ˮ��Ĺ��ɣ�Խ��Խˮ�⣬�������H��������H2SO3>H2CO3>HClO>HCO3����A����ˮ�д���Cl2��H2O
2NaOH��H2����Cl2������2����ȥCa2����Na2CO3����ȥMg2����NaOH����ȥSO42����BaCl2�����Ӳ������������ʣ���̼���ƻ��г�ȥ������BaCl2�����ã���Na2CO3����BaCl2�ĺ��棬���˳����NaOH��BaCl2��Na2CO3����BaCl2��NaOH��Na2CO3����BaCl2��Na2CO3��NaOH��Ȼ����ˣ��ټ������ᣬ��ѡ��B��ȷ����3������������һ�����缫��ӦʽΪ2H2O��2e��=H2����2OH�������a%>b%��ͨ����һ������O2��2H2O��4e��=4OH���������c%>a%,�����c%>a%>b%����4����������ˮ��Ĺ��ɣ�Խ��Խˮ�⣬�������H��������H2SO3>H2CO3>HClO>HCO3����A����ˮ�д���Cl2��H2O![]() HCl��HClO������NaHCO3������NaHCO3��HCl=NaCl��CO2����H2Ŷ,NaHCO3��������ᷴӦ����ʹƽ�����ƣ�HClO��Ũ������A��˵����ȷ��B���Ա�pH��pH���˵��ˮ��̶ȴ�Na2CO3ˮ��̶����B˵������C����������������HClO������������pH���C��˵����ȷ��D���������������������H������Խǿ����Ӧ��������H������Խ������CO32����ǿ����D˵������5��Ũ������HCl�����ʵ���Ϊ100��106��36.5%/36.5mol=106mol��������Ԫ���غ㣬n(NaCl)=n(HCl)=106mol��������Ϊ106��58.5g=58.5��106g����58.5t����6���������̣���Ӧ����ʽΪ2FeTiO3��6C��7Cl2=2FeCl3��2TiCl4��6CO����7����ΪMg��N2��Ӧ�����ɵ���þ����˷�Ӧ����Ϊ3Mg +N2
HCl��HClO������NaHCO3������NaHCO3��HCl=NaCl��CO2����H2Ŷ,NaHCO3��������ᷴӦ����ʹƽ�����ƣ�HClO��Ũ������A��˵����ȷ��B���Ա�pH��pH���˵��ˮ��̶ȴ�Na2CO3ˮ��̶����B˵������C����������������HClO������������pH���C��˵����ȷ��D���������������������H������Խǿ����Ӧ��������H������Խ������CO32����ǿ����D˵������5��Ũ������HCl�����ʵ���Ϊ100��106��36.5%/36.5mol=106mol��������Ԫ���غ㣬n(NaCl)=n(HCl)=106mol��������Ϊ106��58.5g=58.5��106g����58.5t����6���������̣���Ӧ����ʽΪ2FeTiO3��6C��7Cl2=2FeCl3��2TiCl4��6CO����7����ΪMg��N2��Ӧ�����ɵ���þ����˷�Ӧ����Ϊ3Mg +N2![]() Mg3N2��
Mg3N2��

����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ��ˮ | NaBr | ����KI��Һ | �����ԣ�Cl2>Br2>I2 | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | Br2�ı���Һ | ��м | AgNO3��Һ | �����嵥���������������� ����ȡ����Ӧ | |
D | ���� | Na2SO3 | KMnO4��Һ | SO2��ʹKMnO4��Һ��ɫ |
A. A B. B C. C D. D