��Ŀ����
����Ŀ��ʯ���ѻ��ɵõ��л���(CH3)2C===C(CH3)2(������A��ʾ)��
��1��A��ϵͳ����Ϊ________��A��ͨ��״���³�________(�������Һ���̡�)̬��������ΪA����ij�ֹ�����ʹ��ˮ��ɫ����д����صĻ�ѧ����ʽ__________________________��
��2��A��Br2�ļӳɲ���B��NaOH���Ҵ���Һ���ȿ����ɶ�ϩ��C����C�Ľṹ��ʽΪ____________________________________________________________��B����C�ķ�Ӧ����Ϊ________��
��3��C��һ����Br2��Ӧ��������D��E��G����D��HBr�ļӳɲ���ֻ��F����F�Ľṹ��ʽΪ______________________________________________________��
���𰸡� 2,3-����-2-��ϩ Һ (CH3)2C===C(CH3)2 + Br2 ![]() (CH3)2CBrCBr(CH3)2
(CH3)2CBrCBr(CH3)2 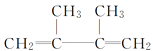 ��ȥ��Ӧ
��ȥ��Ӧ 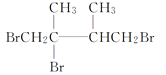
��������(1)����̼̼˫�����������4��C��̼̼˫������2��3��C�м䣬2��3��C����2������A��ϵͳ������Ϊ2��3-����-2-��ϩ��A���ں���6��̼ԭ�ӵ�����������ΪҺ�壬A�к���̼̼˫�����ܹ�����ˮ�����ӳɷ�Ӧ����Ӧ�ķ���ʽΪ��(CH3)2C=C(CH3)2 + Br2 �� (CH3)2CBrCBr(CH3)2�ʴ�Ϊ��2��3-����-2-��ϩ��Һ��(CH3)2C=C(CH3)2 + Br2 �� (CH3)2CBrCBr(CH3)2��
(2)BΪ(CH3)2CBrCBr(CH3)2���������NaOH���Ҵ���Һ���ȿ����ɶ�ϩ����Ϊ��ȥ��Ӧ������CΪ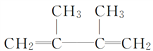 ���ʴ�Ϊ��
���ʴ�Ϊ��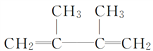 ����ȥ��Ӧ��
����ȥ��Ӧ��
(3)C��һ����Br2��Ӧ��������D��E��G����D��HBr�ļӳɲ���ֻ��F��˵��D�ṹ�Գƣ���C����1��4�ӳ�����D����FΪ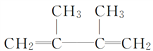 ���ʴ�Ϊ��
���ʴ�Ϊ��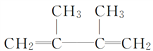 ��
��