��Ŀ����
����Ŀ���������ԭ�����ش����и�С�⣺
(1)25��ʱ��ijFeCl3��Һ��pH=2�������Һ����ˮ���������c(OH-)=___________�������ӷ���ʽ��ʾFeCl3��Һ���ھ�ˮ��ԭ����______________________��
(2)��֪NaHSO4��ˮ�еĵ��뷽��ʽ NaHSO4 �� Na+��H+��SO42-����NaHSO4��Һ��c(H+)______c(OH-)��c(SO42-)(����>��������������<����ͬ)���������� ��������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ��������Ӧ����Һ��pH______7
(3)�����±��ṩ�����ݣ�
��ѧʽ | ���볣�� |
HClO | Ka��3��10-8 |
H2CO3 | Ka1��4.3��10-7 |
Ka2��5.6��10-11 |
��84����Һ(��Ч�ɷ�ΪNaClO)�����ڿ����л�ʧЧ��д��������Ӧ�����ӷ���ʽ�� _____________________________________________
���ж��ڵ�Ũ�ȵ�NaClO��NaHCO3�����Һ�У���������Ũ���ɴ�С��˳�� ____________
���𰸡�10-2mol/L Fe3++3H2O![]() Fe(OH)3+3H+ = > ClO-��H2O��CO2��HClO��HCO3- 2HClO
Fe(OH)3+3H+ = > ClO-��H2O��CO2��HClO��HCO3- 2HClO![]() 2H+��2Cl-��O2�� c(Na+)>c(HCO3-)>c(ClO-)>c(OH-)>c(H+)>c(CO32-)
2H+��2Cl-��O2�� c(Na+)>c(HCO3-)>c(ClO-)>c(OH-)>c(H+)>c(CO32-)
��������
��1��ˮ�������c��OH-��=c��H+����Fe3+������ˮ�����������������壬������������ԣ��Ӷ���ˮ��
��2�����ݵ���غ�������غ�ȷ��c��H+����c��OH-��+c��SO42-���Ĺ�ϵ�����ݻ����Һ�е�����ȷ����Һ������ԣ�
��3�����ɱ��е���ƽ�ⳣ�������ݿ�֪������H2CO3��HClO��HCO3-���Ӷ�ȷ��������Ͷ�����̼��ˮ��Ӧ���������������ȶ��������ֽ⣬�Ӷ�����84����ҺʧЧ��
�������Ӳ�ˮ�⣬����Ũ�������̼��������Ӻʹ����������ˮ��̶�ȷ����Ũ�ȴ�С�������ˮ�������ģ������������Ũ�ȴ�������������Ũ�ȣ�������Һ�������ȷ��������Ũ�Ⱥ�����������Ũ�ȵĹ�ϵ������������Դȷ��������Ũ�Ⱥ�̼�������Ũ�ȵĹ�ϵ��
��1���Ȼ�����ǿ�������Σ���Һ�������Ӿ���ˮ������ģ�ˮ�������c��OH-��=c��H+��=10-2mol/L���Ȼ���ˮ�����������������壬ˮ������ӷ���ʽΪFe3++3H2O![]() Fe(OH)3+3H+��������������ԣ�������ˮ�е�����������ܾ�ˮ���ʴ�Ϊ��10-2mol/L��Fe3++3H2O
Fe(OH)3+3H+��������������ԣ�������ˮ�е�����������ܾ�ˮ���ʴ�Ϊ��10-2mol/L��Fe3++3H2O![]() Fe(OH)3+3H+��
Fe(OH)3+3H+��
��2�����ݵ���غ��c��H+��+C��Na+��=c��OH-��+2c��SO42-�������������غ��C��Na+��=c��SO42-��������c��H+��=c��OH-��+c��SO42-��������������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ��������Ӧ�Ļ�ѧ����ʽΪNaHSO4+Ba��OH��2=BaSO4��+H2O+NaOH�������Һ�е��������������ƣ�������Һ�ʼ��ԣ���Һ��pH��7���ʴ�Ϊ��=������
��3�����ɱ��е���ƽ�ⳣ�������ݿ�֪������H2CO3��HClO��HCO3-������ǿ���Ʊ����ᣬ84����Һ����Ч�ɷ�ΪNaClO�����տ����еĶ�����̼���ɴ�������̼�����ƣ���Ӧ���ӷ���ʽΪ��ClO-+H2O+CO2=HClO+HCO3-�����ɵĴ�����ֽ�������������������Ӧ���ӷ���ʽΪ2HClO![]() 2H+��2Cl-��O2�����ʴ�Ϊ��ClO-��H2O��CO2��HClO��HCO3-��2HClO
2H+��2Cl-��O2�����ʴ�Ϊ��ClO-��H2O��CO2��HClO��HCO3-��2HClO![]() 2H+��2Cl-��O2����
2H+��2Cl-��O2����
�ڵ�Ũ�ȵ�NaClO��NaHCO3�����Һ�У�ClO-��HCO3-ˮ�⣬��Һ�ʼ��ԣ�����c��OH-����c��H+������������H2CO3��HClO����ClO-ˮ��̶ȸ���c��HCO3-����c��ClO-����̼������ʹ���������ӵ�ˮ�������ģ�����c��ClO-����c��OH-����ˮ��̼��������Ӷ��ܵ���������ӣ�̼�������ֻ��̼��������ӵ��룬����c��H+����c��CO32-�������������Ũ���ɴ�С��˳��c(Na+)>c(HCO3-)>c(ClO-)>c(OH-)>c(H+)>c(CO32-)���ʴ�Ϊ��c(Na+)>c(HCO3-)>
c(ClO-)>c(OH-)>c(H+)>c(CO32-)��

����Ŀ������V��Ϊ����Ԫ�أ����γɶ��̬�����ȫ��Һ�������һ�����͵���ɫ��������ϵͳ������ԭ������ͼ��
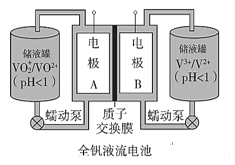
��֪��
�������� | VO2+ | VO2+ | V3+ | V2+ |
��ɫ | ��ɫ | ��ɫ | ��ɫ | ��ɫ |
��1��ȫ��Һ����طŵ�ʱV2+����������Ӧ���õ�طŵ�ʱ�ܷ�Ӧʽ��_______
��2������ɴ���ʱ��������Һ����ɫ�� __________
��3�����ӽ���Ĥ��������_________
��4��������ˮ�����ˮ����Ⱦ���Ժ�����ˮ����VO2+�⣬����Fe3+�ȣ������ۺϴ�����ʵ�ַ���Դ�Ļ������ã��������£�
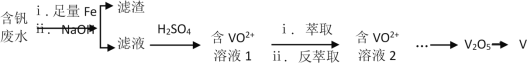
��֪��Һ����Բ�ͬ��Ԫ�صĴ�����ʽ��ͬ��
���Ļ��ϼ� | ���� | ���� |
+4�� | VO2+ | VO(OH)3- |
+5�� | VO2+ | VO43- |
����Һ�з�Ԫ�ص���Ҫ������ʽΪ_______
�������ڿ������ɻҰ�ɫת��Ϊ���ɫ���û�ѧ�����ʾ����NaOH�����ɳ����ķ�Ӧ����_______________��____________��
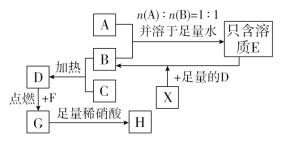
����ȡ������ȡ��ʵ�ַ��ķ�����������̿ɼ�ʾΪ��HAΪ�л���ȡ������
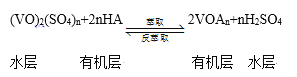
��ȡʱ��������������ԭ���� __________
�������������ε�ⷨ�������ַ��������ʣ���____����
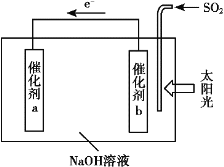
����Ŀ��ʯ��ɽ�д�����ɽˮ�ֳ�����������о�NOx��SO2�ȴ�����Ⱦ������ƴ���������Ҫ���塣
(1)SO2���ŷ���Ҫ������ú��ȼ�գ���ҵ�ϳ��ð�ˮ���շ�����β���е�SO2��
��֪���չ�������ط�Ӧ���Ȼ�ѧ����ʽ���£�
��SO2(g)+NH3��H2O(aq)= NH4HSO3(aq) ��H1=a kJ/mol��
��NH3��H2O(aq)+ NH4HSO3(aq)=(NH4)2SO3(ag)+H2O(l) ��H2=b kJ/mol��
��2(NH4)2SO3(aq)+O2(g)=2(NH4)2SO4(aq) ��H3=c kJ/mol��
��Ӧ2SO2(g)+4NH3��H2O(aq)+O2(g) =2(NH4)2SO4(aq)+2H2O(l)����H=______kJ/mol��
(2)ȼú���糧�����÷�Ӧ2CaCO3(s)+2SO2(g)+O2(g)![]() 2CaSO4(s)+2CO2(g) ��H =681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪTKʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
2CaSO4(s)+2CO2(g) ��H =681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪTKʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
ʱ��/min Ũ��/mol��L1 | 0 | 10 | 20 | 30 | 40 | 50 |
O2 | 1.00 | 0.79 | 0.60 | 0.60 | 0.64 | 0.64 |
CO2 | 0 | 0.42 | 0.80 | 0.80 | 0.88 | 0.88 |
��0��10 min�ڣ�ƽ����Ӧ����v(SO2)=_____mol/(L��min)��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⡣�����ϱ��е������жϣ��ı������������_____������ĸ����
A��ͨ��һ������O2 B������һ�����ķ�״̼���
C���ʵ���С��������� D��������ʵĴ���
(3)NOx���ŷ���Ҫ����������β�����������÷�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��
N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��
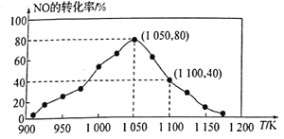
��ͼ��֪��1050Kǰ��Ӧ��NO��ת�������¶����{��������ԭ��Ϊ_______����1100Kʱ��CO2���������Ϊ______��
(4)��ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��������Kp������1050K��1.1��106 Paʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=____[��֪�������ѹ(P��)=������ѹ(P)���������]��
(5)����β���������÷�Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H=746.8 kJ/mol��ʵ���ã�v��=k����c2(NO)��c2(CO)��v��=k����c(N2)��c2(CO2)��k����k��Ϊ���ʳ�����ֻ���¶��йأ���
N2(g)+2CO2(g) ��H=746.8 kJ/mol��ʵ���ã�v��=k����c2(NO)��c2(CO)��v��=k����c(N2)��c2(CO2)��k����k��Ϊ���ʳ�����ֻ���¶��йأ���
�ٴﵽƽ��������¶ȣ�k������ı���____���>����<����=����k������ı�����
������1L���ܱ������г���1 molCO��1 mol NO����һ���¶��´ﵽƽ��ʱ��CO��ת����Ϊ40%����k���Uk��=_____��
����Ŀ��ij��ѧ��ȤС��������ͼװ����ȡ������̽���������й����ʣ�

(1)װ��A����ƿ���Լ���ѡ��________(�����)��
a.Ũ���ᡡb.��ʯ�ҡ�c.���������ס�d.NaOH����
(2)��̽���������ܽ��ԣ���״���£�װ��D���ռ��������ر�K1��K2����K3��������Ȫ��ʵ�������________�������Ȫʵ���װ��D�г���Һ�壬��װ��D����Һ�����ʵ���Ũ��Ϊ__________��(������λ��Ч����)
(3)K2�ĵ���ĩ����Ҫ���Ӱ���������װ�ã�������K2�ĵ���ĩ��������ͼ�е�________װ��(����Ţ�)��
��ѡװ��(����ˮ�к���̪��Һ) | ||
|
|
|
�� | �� | �� |
(4)��̽�������Ļ�ԭ�ԣ���ر�K1��K3��K2������������ȡ����������������װ�á�
��D�а�����������Ӧ�������̣�ͬʱ����һ�ֻ�ѧ�����ȶ����嵥�ʣ��÷�Ӧ���������������ܵ��Ƿ���й©����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��β������Cװ�ô�������β���к�������Cl2����Cװ�����������������Ļ�ѧ����ʽΪ_____��