��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ��A��H�ڱ��е�λ�ã�
�� ���� ���� | IA | | 0 | |||||
| 1 | A | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | B | C | D | | |
| 3 | E | | F | G | | | H | |
��1��D��E��F��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��2��B��C��D�ĵ�һ�������ɴ�С��˳��Ϊ_________________________��
 ��3��A��D��E��H�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��3��A��D��E��H�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________�� (4) E��F������������ˮ�������Ӧ�����ӷ���ʽ
(4) E��F������������ˮ�������Ӧ�����ӷ���ʽ  ��
����5����B���γɵĻ�����CH4��CO��CH3OH�У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ���������������
��CO���ӻ�Ϊ�ȵ�����ķ��Ӻ���
 �ӷֱ�Ϊ ������ �����ݵȵ�����
�ӷֱ�Ϊ ������ �����ݵȵ��������Ʋ�CO���ӵĽṹʽ�ɱ�ʾ����������һ��CO���������������Ҽ��������ݼ���
��6������VSEPR����Ԥ��D��H���γɵ�HD4�� ���ӵĿռ乹��Ϊ______________�͡�
(7)��A��B��C��D��EԪ����ɵ�10���������У�����λ������ �� ��
��1��Na��Al��O
(2)N��O��C
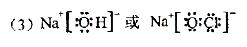
(4)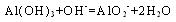
(5) CH4��CH3OH N2 C22�� 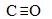 1 2
1 2
��6������������
��7��NH4+ H3O+
����

��ϰ��ϵ�д�
�����Ŀ








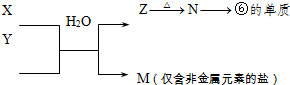
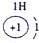
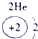
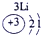
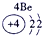
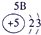
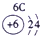
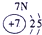
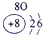
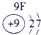
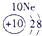
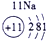





 ��ʾ����
��ʾ����