��Ŀ����
17���������ʣ���Ϊ��ѧ��ѧ�������ʣ�ת����ϵͼ�У�DΪ����ɫ��ĩ��K��һ�ּ���������ˮ�İ�ɫ��״�����һ���ľ�ˮ���ã���Ӧ���������ַ�Ӧ�����δȫ��˵����������ͼʾת����ϵ��д���пհף�
��1��д���������ʵĻ�ѧʽAAl2O3��EFe��GFe��OH��2��
��2��ʵ��������F��Һʱ����Ҫ��������Fe�ֺ����ᣮ
��3��д�����л�ѧ����ʽ�����ӷ���ʽ
A+NaOH��J�����ӷ���ʽΪAl2O3+2OH-=2 AlO2-+H2O��G+B��H�Ļ�ѧ����ʽ4 Fe��OH��2+O2+2H2O=4 Fe��OH��3��
��4�������Һͨ�������ԣ��������ӷ���ʽ����ԭ��Ϊ��Al3++3H2O?Al��OH��3+3H+��
���� ���A�õ�B��C��A�������ᡢ�������Ʒ�Ӧ����AΪAl2O3��DΪ����ɫ��ĩ����C��Ӧ�õ�A��E���÷�ӦΪ���ȷ�Ӧ����DΪFe2O3��CΪAl��BΪO2��EΪFe����ת����ϵ��֪��IΪAlCl3��JΪNaAlO2�����߷�Ӧ�õ�KΪAl��OH��3��E��Fe�������ᷴӦ�õ�FΪFeCl2����GΪFe��OH��2��HΪFe��OH��3��Fe��OH��3�ֽ�õ����������ݴ˽��
��� �⣺���A�õ�B��C��A�������ᡢ�������Ʒ�Ӧ����AΪAl2O3��DΪ����ɫ��ĩ����C��Ӧ�õ�A��E���÷�ӦΪ���ȷ�Ӧ����DΪFe2O3��CΪAl��BΪO2��EΪFe����ת����ϵ��֪��IΪAlCl3��JΪNaAlO2�����߷�Ӧ�õ�KΪAl��OH��3��E��Fe�������ᷴӦ�õ�FΪFeCl2����GΪFe��OH��2��HΪFe��OH��3��Fe��OH��3�ֽ�õ���������
��1��������������֪��AΪAl2O3��EΪFe��GΪFe��OH��2���ʴ�Ϊ��Al2O3��Fe��Fe��OH��2��
��2��FeCl2�ױ���������������Һ�з���ˮ�⣬ʵ��������FeCl2��Һʱ����Ҫ��������Fe�ۺ����ᣬ�ʴ�Ϊ��Fe�ۡ����
��3��A+NaOH��J�����ӷ���ʽΪ��Al2O3+2OH-=2 AlO2-+H2O��
G+B��H�Ļ�ѧ����ʽ��4 Fe��OH��2+O2+2H2O=4 Fe��OH��3��
�ʴ�Ϊ��Al2O3+2OH-=2 AlO2-+H2O��4 Fe��OH��2+O2+2H2O=4 Fe��OH��3��
��4����ΪAlCl3������Һ������ˮ�⣺Al3++3H2O?Al��OH��3+3H+����Һ�����ԣ�
�ʴ�Ϊ��Al3++3H2O?Al��OH��3+3H+��
���� ���⿼�������ƶϣ��漰Fe��AlԪ�ص��ʻ��������ʣ���B����ɫ��C����ǿ�ᡢǿ�Ӧ�����ƶ�ͻ�ƿڣ����ضԻ�ѧ����Ŀ��飬ע�����֪ʶ�����գ�

| A�� | ��һ����������������������Cu��OH��2������Ӧ | |
| B�� | ��������Ҫ�ɷ�����֬�ڼ���������ˮ�����ɵ� | |
| C�� | ���ۡ���ά�غ���֬������Ȼ�߷��ӻ����� | |
| D�� | ��������Һ������ͭ������ij���������������ˮ |
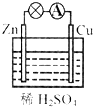
| A�� | ���������ķ�Ӧ��Zn-2e-�TZn2+ | B�� | ������������������Ӧ | ||
| C�� | һ��ʱ�����Һ��pH��С | D�� | һ��ʱ���ͭƬ���������� |
| A�� | �������е���ʽ��ԭ���У���1s22s22p63s23p2����1s22s22p3����1s22s22p2����1s22s22p63s23p4��ԭ�Ӱ뾶�����Ǣ� | |
| B�� | ����ԭ�ӵļ۵����Ų��У���3s23p1��3s23p2��3s23p3��3s23p4��Ӧ�ĵ�һ����������Ǣ� | |
| C�� | ijԪ�ص������ֱܷ�Ϊ738��1451��7733��10540��13630��17995��21703��������������Ӧʱ�������ɵ���������X3+ | |
| D�� | ��Na��K��Rb����N��P��As����O��S��Se����Na��P��Cl Ԫ�صĵ縺����ԭ������������������Ǣ� |
�������Ͻ� ���Ƶ��� ���ƴ��� ������ɫ������ ����������ˮ ��������С�մ���ʳƷ���ɼ� ���������� ������������������θ��ƽ ���װ�ǹ���СʳƷ��
| A�� | �ۢݢ�� | B�� | �ޢ�� | C�� | �ۢݢ� | D�� | �ۢݢޢߢ�� |
| A�� | �Ҵ����ӱ���ģ�ͣ� | |
| B�� | ȩ���ĵ���ʽ�� | |
| C�� |  ������Ϊ��2��4��4-����-1-��ϩ ������Ϊ��2��4��4-����-1-��ϩ | |
| D�� | ���ᡢ�����ǡ����۵����ʽ��ΪCH2O |
| A�� | Aԭ��������������Bԭ�ӵ������������� | |
| B�� | Aԭ�ӵ��Ӳ�����Bԭ�ӵĵ��Ӳ����� | |
| C�� | 1molA�������û����ɵ�H2��1mol B�������û����ɵ�H2�� | |
| D�� | A������������Ӧˮ������ǿ���B������������Ӧˮ���������� |
| A�� | K3C60�м������Ӽ������м��Թ��ۼ� | |
| B�� | ������������״̬���ܵ��� | |
| C�� | �����ʵĻ�ѧʽ��д��KC20 | |
| D�� | 1molK3C60�к��еĹ��ۼ�����ĿԼΪ60��6.02��1023�� |
| A�� | ��Ԫ�ص���������1��2 | B�� | ��Ԫ�ص����ʵ���֮����1��1 | ||
| C�� | N2O4��NO2�ķ�����֮����1��1 | D�� | ��ԭ�Ӹ���֮����2��1 |