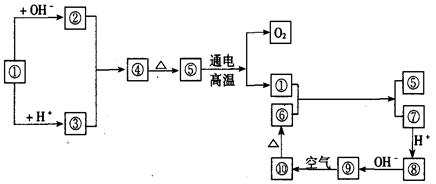
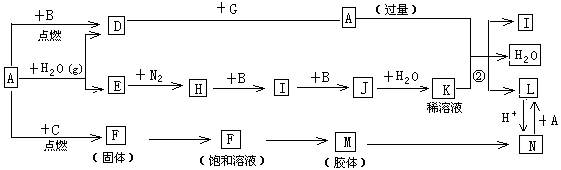
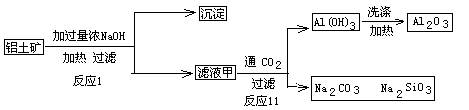
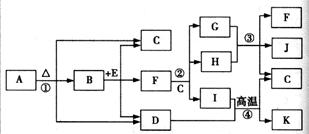
��Ŀ����
��14�֣�����A��B��C��D�������ʣ�B��D����ɫ��Ӧ��Ϊ��ɫ��C��һ����ʽ�Σ���C����BaCl2��Һ�в���������İ�ɫ�������ɣ�D�����ڸ��������������ʧȥ�ᾧˮ��ɰ�ɫ��ĩ����A��B��C��D��������ʵ�飬ʵ����̺ͼ�¼����ͼ��ʾ������������ȥ������ش�

��1��X��B��Ӧ�����ӷ���ʽΪ����������������������������������������������������
��2��D��ҺpHֵ����7��ԭ���ǣ������ӷ���ʽ��ʾ����������������������������������
��3��д��C�����B��Ӧ�����ȣ������ӷ���ʽ����__________________��������������
��4����B��C��ϡ��Һ��Ϻ����ȣ���Һ�����ԣ������Һ������Ũ�ȴӴ�С��˳��_____________________.
��5��Y��ͬ�����ͬ�������з����ȶ�����ǿ�� ����Ҳ�Ƿе���ߵģ� �������ж�������"��"��"��"��
��6����������������B��ϡ��Һ�зֱ��������ϡ���ᡢϡ���ᡢŨ���ᣬ������1molH2Oʱ�ķ�Ӧ�ȷֱ�Ϊ��H1 ����H2����H3 ���������ɴ�С����Ϊ��___________________.

��1��X��B��Ӧ�����ӷ���ʽΪ����������������������������������������������������
��2��D��ҺpHֵ����7��ԭ���ǣ������ӷ���ʽ��ʾ����������������������������������
��3��д��C�����B��Ӧ�����ȣ������ӷ���ʽ����__________________��������������
��4����B��C��ϡ��Һ��Ϻ����ȣ���Һ�����ԣ������Һ������Ũ�ȴӴ�С��˳��_____________________.
��5��Y��ͬ�����ͬ�������з����ȶ�����ǿ�� ����Ҳ�Ƿе���ߵģ� �������ж�������"��"��"��"��
��6����������������B��ϡ��Һ�зֱ��������ϡ���ᡢϡ���ᡢŨ���ᣬ������1molH2Oʱ�ķ�Ӧ�ȷֱ�Ϊ��H1 ����H2����H3 ���������ɴ�С����Ϊ��___________________.
��1�� Al(OH)3+OH�� = AlO2�� +2H2O��2�֣�
��2��CO32��+ H2O HCO3��+OH�� ��2�֣�
HCO3��+OH�� ��2�֣�
��3��NH4+ +H+ +2OH��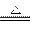 NH3��+2H2O��3�֣�
NH3��+2H2O��3�֣�
��4��C(Na+)��C(SO42��)��C(NH4+ )��C(H+) = C(OH��) ��3�֣�
��5���� ���� ����1�֣� ��6����H1 >��H2 >��H3 (2��)
��2��CO32��+ H2O
 HCO3��+OH�� ��2�֣�
HCO3��+OH�� ��2�֣���3��NH4+ +H+ +2OH��
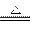 NH3��+2H2O��3�֣�
NH3��+2H2O��3�֣���4��C(Na+)��C(SO42��)��C(NH4+ )��C(H+) = C(OH��) ��3�֣�
��5���� ���� ����1�֣� ��6����H1 >��H2 >��H3 (2��)
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 ��
��