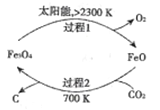
��Ŀ����
����Ŀ��һ���¶��£���1L�����ܱ������м���lmol��N2(g)��3molH2(g)������Ӧ��N2(g)+3H2(g) ![]() 2NH3(g) ��H<0�� NH3�����ʵ�����ʱ��Ĺ�ϵ���±���ʾ������˵���������
2NH3(g) ��H<0�� NH3�����ʵ�����ʱ��Ĺ�ϵ���±���ʾ������˵���������
ʱ��(min) | 0 | t1 | t2 | t3 |
NH3���ʵ���(mol) | 0 | 0.2 | 0.3 | 0.3 |
A. 0t1min��v(NH3)=![]() mol��L-1��min-1
mol��L-1��min-1
B. �����¶ȣ���ʹ����Ӧ���ʼ�С���淴Ӧ��������ƽ������
C. N2(g)+3H2(g) ![]() 2NH3(g)�Ļ��С��2NH3(g)
2NH3(g)�Ļ��С��2NH3(g) ![]() N2(g)+3H2(g)�Ļ��
N2(g)+3H2(g)�Ļ��
D. t3ʱ�ټ���1mol��N2(g)��3molH2(g)����Ӧ����ƽ��ʱ��c(N2)>0.85mol��L-1
���𰸡�B
��������A��0t1min��v(NH3)=  =
=![]() mol��L-1��min-1����A��ȷ��B�������¶ȣ������淴Ӧ���ʾ�����B����C��N2(g)+3H2(g)
mol��L-1��min-1����A��ȷ��B�������¶ȣ������淴Ӧ���ʾ�����B����C��N2(g)+3H2(g) ![]() 2NH3(g)�Ƿ��ȷ�Ӧ��������Ӧ�Ļ��С���淴Ӧ�Ļ�ܣ���C��ȷ��D��1mol��N2(g)��3molH2(g)����Ӧ����ƽ��ʱ��NH3(g)�ı仯��Ϊ0.3mol����N2�ı仯��Ϊ0.15mol��ƽ��ʱc(N2)=0.85mol��L-1���ټ���1mol��N2(g)��3molH2(g)��ƽ�������ƶ���������������ԭ������Ӧ����ƽ��ʱ��c(N2)>0.85mol��L-1����D��ȷ����ΪB��
2NH3(g)�Ƿ��ȷ�Ӧ��������Ӧ�Ļ��С���淴Ӧ�Ļ�ܣ���C��ȷ��D��1mol��N2(g)��3molH2(g)����Ӧ����ƽ��ʱ��NH3(g)�ı仯��Ϊ0.3mol����N2�ı仯��Ϊ0.15mol��ƽ��ʱc(N2)=0.85mol��L-1���ټ���1mol��N2(g)��3molH2(g)��ƽ�������ƶ���������������ԭ������Ӧ����ƽ��ʱ��c(N2)>0.85mol��L-1����D��ȷ����ΪB��