ЬтФПФкШн
ЁОЬтФПЁПЖўбѕЛЏЬМЕФЛиЪеРћгУЪЧЛЗБЃСьгђбаОПШШЕуЁЃ
ЃЈlЃЉдкЬЋбєФмЕФзїгУЯТЃЌвдCO2ЮЊдСЯжЦШЁЬПКкЕФСїГЬШчЭМЫљЪОЃЌзмЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________ЁЃ
ЃЈ2ЃЉЙЄвЕЩЯПЩгУCO2КЭH2ЗДгІжЦЕУМзДМЁЃдк2ЁС105PaЁЂ300ЁцЕФЬѕМўЯТЃЌCO2КЭH2ЗДгІЩњГЩМзДМКЭвКЬЌЫЎЃЌЕБЯћКФ2molCO2ЪБЗХГі98kJЕФШШСПЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
ЃЈ3ЃЉШчРћгУCO2КЭCH4жиећПЩжЦКЯГЩЦјЃЈжївЊГЩЗжЮЊCOЁЂH2ЃЉ, ЦфдРэЮЊЃКCO2(g)+CH4(g)![]() 2CO(g)+2H2(g)ЁЃдкУмБеШнЦїжаЭЈШыЮяжЪЕФСПОљЮЊ1molЕФCH4КЭCO2ЃЌдквЛЖЈЬѕМўЯТЗЂЩњЗДгІЁЃCH4ЕФЦНКтзЊЛЏТЪгыЮТЖШМАбЙЧПЃЈЕЅЮЛPaЃЉЕФЙиЯЕШчЭМЫљЪОЃК
2CO(g)+2H2(g)ЁЃдкУмБеШнЦїжаЭЈШыЮяжЪЕФСПОљЮЊ1molЕФCH4КЭCO2ЃЌдквЛЖЈЬѕМўЯТЗЂЩњЗДгІЁЃCH4ЕФЦНКтзЊЛЏТЪгыЮТЖШМАбЙЧПЃЈЕЅЮЛPaЃЉЕФЙиЯЕШчЭМЫљЪОЃК
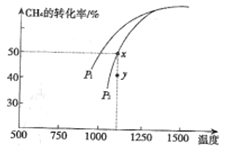
ЂйбЙЧПЃКP1_____P2ЃЈЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБЃЌЯТЭЌ) , yЕуЃКVе§_______VФцЁЃ
ЂквбжЊЦјЬхЗжбЙЃЈPЗжЃЉ=ЦјЬхзмбЙЃЈPзмЃЉЁСЦјЬхЕФЬхЛ§ЗжЪ§ЁЃгУЦјЬхЗжбЙДњЬцЦНКтХЈЖШПЩвдЕУЕНЦНКтГЃЪ§KPЃЌЧѓXЕуЖдгІЮТЖШЯТЗДгІЕФЦНКтГЃЪ§KP=________ЁЃ
ЃЈ4ЃЉNa2CO3ШмвКвВЭЈГЃгУРДВЖЛёCO2ЃЌЗЂЩњШчЯТЗДгІЃКCO32-+CO2+H2O![]() 2HCO3-ЃЌгУ0.12mol/LNa2CO3ШмвКШєЮќЪеCO2вЛЖЮЪБМфКѓЃЌШмвКЕФpH=7ЃЌШмвКжаc(HCO3-)/c(CO32-)=10ЃЌдђШмвКжаЕФc(CO32-) =__________ЁЃ
2HCO3-ЃЌгУ0.12mol/LNa2CO3ШмвКШєЮќЪеCO2вЛЖЮЪБМфКѓЃЌШмвКЕФpH=7ЃЌШмвКжаc(HCO3-)/c(CO32-)=10ЃЌдђШмвКжаЕФc(CO32-) =__________ЁЃ
ЃЈ5ЃЉМзДМШМСЯЕчГиЃЈМђГЦDMFCЃЉПЩзїЮЊГЃЙцФмдДЕФЬцДњЦЗЖјБИЪмЙизЂЁЃDMFCЕФЙЄзїдРэШчЭМЫљЪОЃК
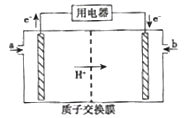
ЂйМгШыaЮяжЪЕФЕчМЋЪЧЕчГиЕФ______ЃЈЬюЁАе§ЁБЛђЁАИКЁБЃЉМЋЃЌЦфЕчМЋЗДгІЪНЮЊ_______ЁЃ
ЂкГЃЮТЯТвдИУзАжУзїЕчдДЃЌгУЧщадЕчМЋЕчНтNaClКЭCuSO4ЕФЛьКЯШмвКЃЌЕБЕчТЗжаЭЈЙ§0.4 mol ЕчзгЕФЕчСПЪБЃЌСНЕчМЋОљЕУЕН0.14molЕФЦјЬхЁЃШєЕчНтКѓШмвКЬхЛ§ЮЊ40LЃЌдђЕчНтКѓШмвКЕФpH=_____ЁЃ
ЁОД№АИЁП CO2 ![]() C+O2 CO2(g)+3H2(g)=CH3OH(l)+H2O(l) ЁїHЃН-49kJЁЄmol-1 ЃМ >
C+O2 CO2(g)+3H2(g)=CH3OH(l)+H2O(l) ЁїHЃН-49kJЁЄmol-1 ЃМ > ![]() Pa2 0.02mol/L ИК CH3OH+H2O-6e-=CO2+6H+ 11
Pa2 0.02mol/L ИК CH3OH+H2O-6e-=CO2+6H+ 11
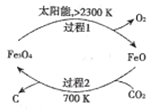
ЁОНтЮіЁП(l)дк700KЬѕМўЯТЃЌCO2КЭFeOЗЂЩњЗДгІЩњГЩCКЭFe3O4ЃЌЙ§ГЬ1жаFe3O4ЗжНтЩњГЩFeOКЭO2ЃЌЫљвдећИіЗДгІЙ§ГЬжаFeOзїДпЛЏМСЃЌИљОнЗДгІЮяКЭЩњГЩЮяМАЗДгІЬѕМўЪщаДЗНГЬЪНЮЊCO2 ![]() C+O2ЃЛ
C+O2ЃЛ
(2)дк2ЁС105PaЁЂ300ЁцЕФЬѕМўЯТЃЌCO2КЭH2ЗДгІЩњГЩМзДМКЭЫЎЃЌЕБЯћКФ2molCO2ЪБЗХГі98kJЕФШШСПЃЌЯћКФ1molCO2ЪБЃЌЗДгІЗХГіШШСП49kJЃЌдђИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊЃКCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)ЁїH=-49kJ/molЃЛ
CH3OH(g)+H2O(g)ЁїH=-49kJ/molЃЛ
(3)ЂйИУЗДгІе§ЯђЮЊЬхЛ§діДѓЕФЗНЯђЃЌбЙЧПдНДѓЃЌCH4ЕФзЊЛЏТЪдНаЁЃЌвбжЊЯрЭЌЮТЖШЯТЃЌP1ЬѕМўЯТЕФзЊЛЏТЪДѓгкP2ЃЌдђP1ЃМP2ЃЛбЙЧПЮЊP2ЪБЃЌдкYЕуЗДгІЮДДяЕНЦНКтЃЌдђЗДгІе§ЯђвЦЖЏЃЌЫљвдv(е§)ЃОv(Фц)ЃЛ
Ђк CO2(g)+CH4(g)![]() 2CO(g)+2H2(g)
2CO(g)+2H2(g)
Ц№ЪМЮяжЪЕФСП(mol) 1 1 0 0
БфЛЏЮяжЪЕФСП(mol) 0.5 0.5 1 1
ЦНКтЮяжЪЕФСП(mol) 0.5 0.5 1 1
дђЦНКтЪБЃКCO2(g)ЕФЗжбЙЮЊ![]() Pзм=
Pзм=![]() PзмЃЌCH4(g) ЕФЗжбЙЮЊ
PзмЃЌCH4(g) ЕФЗжбЙЮЊ![]() PзмЃЌCO(g)ЕФЗжбЙЮЊ
PзмЃЌCO(g)ЕФЗжбЙЮЊ![]() PзмЃЌH2(g)ЕФЗжбЙЮЊ
PзмЃЌH2(g)ЕФЗжбЙЮЊ![]() PзмЃЌXЕуЖдгІЮТЖШЯТЗДгІЕФЦНКтГЃЪ§KP=
PзмЃЌXЕуЖдгІЮТЖШЯТЗДгІЕФЦНКтГЃЪ§KP=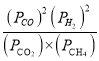 =
=![]() Pa2ЃЛ
Pa2ЃЛ
(4)ЩшШмвКжаЕФc(CO32-) ЮЊcmol/LЃЌдђЗДгІжаМѕаЁЕФc(CO32-) ЮЊ(0.12-c)mol/LЃЌЩњГЩЕФc(HCO3-)ЮЊ2(0.12-c)mol/LЃЌИљОнc(HCO3-)/c(CO32-)=10ЃЌМД2(0.12-c)ЁТc=10ЃЌНтЕУЃКc=0.02ЃЌМДзюжеШмвКжаЕФc(CO32-)ЮЊ0.02mol/LЃЛ
(5)ЂйИљОнзАжУЭМЗжЮіЃЌaЕчМЋДІЕчзгСїГіЃЌзідЕчГиИКМЋЃЌЮяжЪЗЂЩњбѕЛЏЗДгІЃЌИКМЋЮЊМзДМЪЇШЅЕчзгЃЌПМТЧЕНгаH+зЊвЦЕНе§МЋДІЃЌдђЕчМЋВњЮяЮЊCO2КЭH+ЃЌдђЦфЕчМЋЗДгІЪНЮЊЃКCH3OH+H2O-6e-ЈTCO2+6H+ЃЛ
ЂкЖшадЕчМЋЕчНтNaClКЭCuSO4ЕФЛьКЯШмвКЃЌЕчНтЪБЃЌШмвКжабєРызгЯђвѕМЋвЦЖЏЃЌвѕРызгЯђбєМЋвЦЖЏЃЌИљОнЗХЕчЫГађЃЌвѕМЋЗХЕчЫГађЮЊЃКCu2+ЃЌNa+ЃЌбєМЋЗХЕчЫГађЮЊЃКCl-ЃЌSO42-ЃЌЕБЕчТЗжаЭЈЙ§0.4molЕчзгЕФЕчСПЪБЃЌСНЕчМЋОљЕУЕН0.14molЕФЦјЬхЃЌбєМЋПЩвдЕУЕНCl2ЃЌвѕМЋжЛПЩвдЕУЕНH2ЃЌбєМЋПЩвдВњЩњCl2КЭO2ЃЌвѕМЋЕУЕН0.14molH2ЃЌзЊвЦЕФЕчзгЪ§ЮЊ0.14molЁС2=0.28molЃЌгЩгкЕчТЗжаЭЈЙ§0.4molЕчзгЃЌдђЕчНтCu2+ЪБзЊвЦЕчзгЪ§ЮЊ0.4mol-0.28mol=0.12molЃЌбєМЋПЩвдВњЩњCl2КЭO2ЃЌВњЩњетСНжжЦјЬхЕФЮяжЪЕФСПвЛЙВЮЊ0.14molЃЌзЊвЦЕФЕчзгЪ§ЮЊ0.4molЃЌдђгаЙиЯЕЃКn(Cl2)+n(O2)ЃН0.14molЁЂ2n(Cl2)+4n(O2)ЃН0.4molЃЌНтЕУЃКn(Cl2)ЃН0.08molЁЂn(O2)ЃН0.06molЃЌВњЩњ0.06molO2ЃЌH2ВњЩњгЩЫЎЗХЕчЖјРДЃЌЕчМЋЗДгІЮЊЃК2H2O+2e-ЈTH2Ёќ+2OH-ЃЌВњЩњ0.14molH2ЃЌШмвКжаЩњГЩ0.28molOH-ЃЌбєМЋВњЩњO2гЩOH-ЗХЕчЖјРДЃЌЕчМЋЗДгІЮЊЃК4OH--4e-ЈTO2Ёќ+2H2OЃЌВњЩњ0.06molO2ЯћКФ0.24molOH-ЃЌдђШмвКжаЪЃгрOH-ЕФЮяжЪЕФСПЮЊ0.28mol-0.24mol=0.04molЃЌЕчНтКѓШмвКЕФЬхЛ§ЮЊ40LЃЌдђШмвКжаc(OH-)=![]() =0.001mol/LЃЌдђШмвКжаpOH=-lgc(OH-)=3ЃЌЫљвдШмвКpH=14-pOH=11ЁЃ
=0.001mol/LЃЌдђШмвКжаpOH=-lgc(OH-)=3ЃЌЫљвдШмвКpH=14-pOH=11ЁЃ

ЁОЬтФПЁПЛиД№ЯТСаЮЪЬт
ЃЈ1ЃЉЯжгаЯТСаЪЎжжЮяжЪЃКЂйO2ЃЛЂкFeЃЛЂлCaOЃЛЂмCO2ЃЛЂнH2SO4ЃЛЂоBaЃЈOHЃЉ2ЃЛЂпКьКжЩЋЕФЧтбѕЛЏЬњНКЬхЃЛЂрСђЫсФЦШмвКЃЛЂсЯЁЯѕЫсЃЛЂтCu2ЃЈOHЃЉ2CO3 ЃЎ ЃЈiЃЉАДЮяжЪЕФЪїзДЗжРрЗЈЬюаДБэИёЕФПеАзДІЃК
ЗжРрБъзМ | Н№ЪєЕЅжЪ | бѕЛЏЮя | ШмвК | НКЬх |
ЪєгкИУРрЕФЮяжЪ | Ђк | ЂрЂс |
ЃЈiiЃЉЩЯЪіЮяжЪжаЪєгкЗЧЕчНтжЪЕФгаЃЛЩЯЪіЮяжЪжаФмгыбЮЫсЗДгІЕФЕчНтжЪгаЃЈЬюађКХЃЉЃЎ
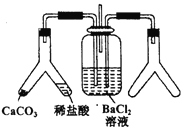
ЃЈ2ЃЉЃЈiЃЉдк KClO3+6HClЃЈХЈЃЉ=KCl+3Cl2Ёќ+3H2O ЕФЗДгІжаЃЌбѕЛЏМСЪЧ ЃЌ ЛЙдВњЮяЪЧ ЃЌ бѕЛЏВњЮягыЛЙдВњЮяЕФжЪСПБШЪЧ ЃЎ ЃЈiiЃЉдкЗДгІMnO2+4HCl=MnCl2+Cl2Ёќ+2H2OжаЃЌУПЩњГЩБъзМзДПіЯТ4.48LCl2 ЃЌ зЊвЦЕФЕчзгЕФЮяжЪЕФСПЮЊmolЃЎ
ЁОЬтФПЁПРћгУзъдќ[КЌCo(OH)2ЁЂFe2O3ЁЂAl2O3ЁЂMnOЕШ)ПЩвдРДжЦШЁзъЕФбѕЛЏЮяКЭCoCl2ЁЄ6H2OЃЌЙЄвеСїГЬШчЭМЫљЪОЃК
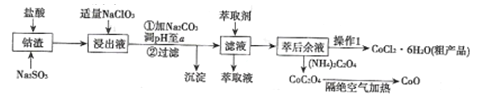
ЂйВПЗжбєРыгквдЧтбѕЛЏЮяаЮЪНГСЕэЪБШмвКЕФpHМћЯТБэЃКЃЈН№ЪєРызгХЈЖШЮЊЃК0.01mol/L)
ГСЕэЮя | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
ПЊЪМГСЕэ | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
ЭъШЋГСЕэ | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
ЂкCoCl2ЁЄ6H2OШлЕуЮЊ86ЁцЃЌМгШШжС110ЁЋ120ЁцЪБЃЌЪЇШЅНсОЇЫЎЩњГЩЮоЫЎТШЛЏюмЁЃ
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉНўГівККЌгаЕФбєРызгжївЊгаH+ЁЂCo2+ЁЂFe2+ЁЂMn2+ЁЂA13+ЕШЃЌНўГіЙ§ГЬжаCo(OH)3ЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ________ЁЃ
ЃЈ2ЃЉСїГЬжаМгШЫЪЪСПЕФNaClO3ЕФзїгУЪЧ___________ЁЃШєМгШыNaClO3Й§СПЃЌПЩФмВњЩњгаЖОЦјЬхЃЌаДГіЩњГЩИУгаЖОЦјЬхЕФРызгЗНГЬЪН___________________ЁЃ
ЃЈ3ЃЉМгШыNa2CO3ЕїНкШмвКЕФpH=a,aЕФЗЖЮЇзюКУЪЧ___________ЁЃГСЕэЕФжївЊГЩЗжЪЧ______ЁЃ
ЃЈ4ЃЉнЭШЁМСЖдН№ЪєРызгЕФнЭШЁТЪгыpHЕФЙиЯЕШчЭМЫљЪОЁЃЯђЁАТЫвКЁБжаМгШынЭШЁМСЕФФПЕФЪЧ________ЃЛЦфЪЙгУЕФзюМбpHЗЖЮЇЪЧ________ЁЃ
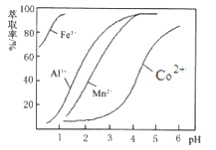
A. 2.0ЁЋ2.5 B. 3.0ЁЋ3.5 C. 4.0ЁЋ4.5 D. 5.0ЁЋ5.5
ЃЈ5ЃЉЁАВйзї1ЁБжаАќКЌ3ИіЛљБОЪЕбщВйзїЃЌЫќУЧвРДЮЪЧ______ЁЂ______КЭЙ§ТЫЁЃ
ЃЈ6ЃЉИєОјПеЦјМгШШCoC2O4ЩњГЩCoOКЭЬМЕФбѕЛЏЮяЃЌЖдгІЕФЛЏбЇЗНГЬЪНЮЊ_______________ЁЃ
ЁОЬтФПЁПвЛЖЈЮТЖШЯТЃЌдк1LКуШнУмБеШнЦїжаМгШыlmolЕФN2(g)КЭ3molH2(g)ЗЂЩњЗДгІЃКN2(g)+3H2(g) ![]() 2NH3(g) ЁїH<0ЃЌ NH3ЕФЮяжЪЕФСПгыЪБМфЕФЙиЯЕШчЯТБэЫљЪОЃЌЯТСаЫЕЗЈДэЮѓЕФЪЧ
2NH3(g) ЁїH<0ЃЌ NH3ЕФЮяжЪЕФСПгыЪБМфЕФЙиЯЕШчЯТБэЫљЪОЃЌЯТСаЫЕЗЈДэЮѓЕФЪЧ
ЪБМф(min) | 0 | t1 | t2 | t3 |
NH3ЮяжЪЕФСП(mol) | 0 | 0.2 | 0.3 | 0.3 |
A. 0t1minЃЌv(NH3)=![]() molЁЄL-1ЁЄmin-1
molЁЄL-1ЁЄmin-1
B. Щ§ИпЮТЖШЃЌПЩЪЙе§ЗДгІЫйТЪМѕаЁЃЌФцЗДгІЫйТЪдіДѓЃЌЙЪЦНКтФцвЦ
C. N2(g)+3H2(g) ![]() 2NH3(g)ЕФЛюЛЏФмаЁгк2NH3(g)
2NH3(g)ЕФЛюЛЏФмаЁгк2NH3(g) ![]() N2(g)+3H2(g)ЕФЛюЛЏФм
N2(g)+3H2(g)ЕФЛюЛЏФм
D. t3ЪБдйМгШы1molЕФN2(g)КЭ3molH2(g)ЃЌЗДгІДяаТЦНКтЪБЃЌc(N2)>0.85molЁЄL-1