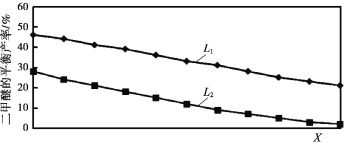
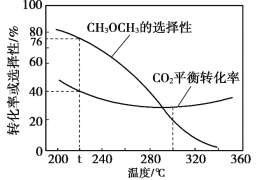
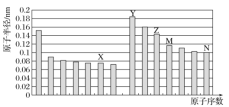
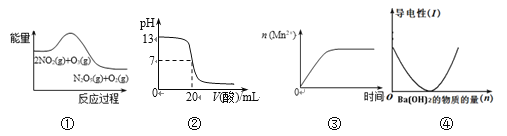
��Ŀ����
����Ŀ�����ܱ������н��з�ӦCH4(g)+H2O(g) ![]() CO(g)+3H2(g)�� ��H>0�����c(CH4)�淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������ж���ȷ���ǣ� ��
CO(g)+3H2(g)�� ��H>0�����c(CH4)�淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������ж���ȷ���ǣ� ��
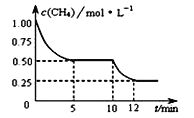
A. 10 minʱ���ı��������������������¶�
B. ��Ӧ���е�12minʱ��CH4��ת����Ϊ25%
C. 0��5 min�ڣ�v(H2)��0.1 mol��(L��min)-1
D. �����£���С���������ƽ���H2Ũ�ȼ�С
���𰸡�A
��������
A. ��ͼ��֪��10min ʱ�����Ũ�ȼ�����С���÷�Ӧ������Ӧ�����ƶ����÷�Ӧ����Ӧ�����ȷ�Ӧ�������������¶ȣ���A��ȷ��
B. ��Ӧ���е�12minʱ��CH4��ת����Ϊ![]() ��100��=75������B����
��100��=75������B����
C. ����ͼ��֪��ǰ5min�ڼ����Ũ����1.00mol/ L��СΪ0.50mol/L����v(CH4) =(1-0.5)molL-1/5min=0.1mol/ (Lmin) ���ɻ�ѧ������֮�ȵ��ڷ�Ӧ����֮�ȣ���v (H2)=3��0.1mol/ (Lmin) =0. 3mol/(Lmin) ����C����
D. �����£���С���������������Ũ��������Ȼѹǿ����ƽ�����淴Ӧ�����ƶ����ƶ��Ľ����С����Ũ�ȵ�����������������ԭ������������Ũ������ƽ���c (H2)����D����
��ѡA��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����б����еĸ����������������ͼ�����߱�ʾ���� �� ��
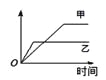
������ Ӧ | ������ | �� | �� | |
A | ��ͬ�����İ�����ͬһ������ | ������ת���� | 500�� | 400�� |
B | �������ء��Ʒֱ�������ˮ��Ӧ | H2���� | �� | �� |
C | ������ɱ�ĺ�ѹ�����У������1�U3��N2��H2�� | ������Ũ�� | ���ԸߵĴ��� | ����һ��Ĵ��� |
D | 2 molSO2��1 molO2������ͬ�¶���2SO2+ O2 | SO3���ʵ��� | 2������ѹ | 10������ѹ |
A. AB. BC. CD. D
����Ŀ������ʵ�ˮ��Һ�д��ڵ���ƽ�⡣
��1�������dz��������ᡣ
�ٴ�����ˮ��Һ�еĵ��뷽��ʽΪ____________��
�����з����У�����ʹ������Һ��CH3COOH����̶��������_______������ĸ��ţ���
a �μ�����Ũ���� b ����Һ
c ��ˮϡ�� d �������������ƾ���
��2����0.1 mol��L-1 NaOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1 mol��L-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߡ�
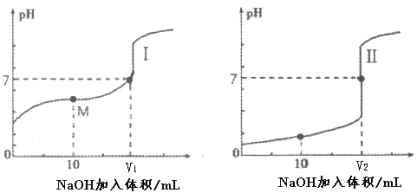
�ٵζ������������___________������I��������������
�ڵζ���ʼǰ��0.1 mol��L-1 NaOH��0.1 mol��L-1�������0.1 mol��L-1����������Һ����ˮ�������c(H+)������_______��Һ��
��ͼI�У�V=10ʱ����Һ�е�c(H+)_________c(OH-) ������>��������������<������ͬ����c(Na+)_________c(CH3COO-)��
��3���±�Ϊijͬѧ����25��ʱ���ס���������Һ��pH��
�� | �� | |
pH | 11 | 11 |
��Һ | ��ˮ | ����������Һ |
�ټ���Һ�е�c(OH-) =___________mol/L��
��25��ʱ��������ļס�������Һ���Ũ�ȵ����ᷴӦ�����ĵ������������_____�ҡ�
�ۼס�����Һ����ˮϡ��10����������Һ��pH����_____�ҡ�
��4��ú̿��ҵ�о������������SO2��Ϊ��ֹ��Ⱦ���������������շ����д������������ʿ���������SO2����____________��
A H2O2 B Na2CO3 C Na2SO3 D Ca(OH)2
��5���±��Ǽ��ֳ�������ĵ��볣��
��ѧʽ | CH3COOH | H2SO3 | HClO | H2CO3 |
���� ���� | 1.8��10-5 | K1=1.23��10-2 K2=6.6��10-8 | 3.0��10-8 | K1=4.4��10-7 K2=4.7��10-11 |
/span>
���·�Ӧ��Ӧ�����ӷ���ʽ��ȷ����___________��
A Na2CO3��Һ��������SO2��CO32-+SO2+H2O = SO32-+HCO3-
B Ư��Һ��Ч��ԭ����ClO-+CO2+H2O = HClO+HCO3-
C ����������Һ��������SO2��ClO-+SO2+H2O = HClO+SO32-
D �����ˮ���е�CaCO3��2CH3COOH+CaCO3 = Ca2++2CH3COO-+H2O+CO2��