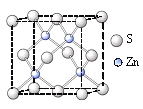
��Ŀ����
����Ŀ��X��Y��Z��������̬���ʣ���һ���¶�����仯������ͼ������˵��һ����ȷ���ǣ� ��
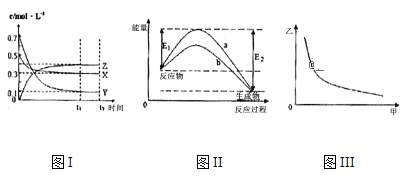
A.�÷�Ӧ���Ȼ�ѧ����ʽΪX(g) +3Y(g) 2Z(g) ��H= -��E2-E1��kJ
B.��ͼIII�мױ�ʾѹǿ���ұ�ʾZ�ĺ���������仯����ͼIII������
C.���¶��£���Ӧ��ƽ�ⳣ����ֵԼΪ533���������¶ȣ��÷�Ӧ��ƽ�ⳣ����С��Y��ת���ʽ���
D.ͼII������b�Ǽ������ʱ�������仯���ߣ�����a��û�м������ʱ�������仯����
���𰸡�C
��������
A. ͼ����X��Y�����ʵ���Ũ����С��Ӧ�Ƿ�Ӧ�Z�����ʵ���Ũ��������Ӧ�������Ũ�ȵı仯��ֵΪ��0.5-0.3������0.7-0.1������0.4-0��=1��3��2������Ũ�ȵı仯֮�ȵ��ڻ�ѧ������֮�ȿ�֪��Ӧ����ʽӦΪX(g) +3Y(g) 2Z(g)����ͼ����֪��Ӧ������������������������������Ӧ���ȣ���Ӧ��Ϊ��H= -��E2-E1��kJ/mol���÷�Ӧ�Ȼ�ѧ����ʽΪX(g) +3Y(g) 2Z(g) ��H= -��E2-E1��kJ/mol����Ӧ�ȵĵ�λΪkJ/mol��A�����
B. ѹǿ����ƽ�����ƣ�Z�ĺ���Ӧ����B�����
C. �÷�Ӧ���ȣ������¶�ƽ�����ƣ��÷�Ӧ��ƽ�ⳣ����С��Y��ת���ʽ��ͣ�C����ȷ��
D. ͼ��Ҳ����Ϊ���벻ͬ�Ĵ�����b�Ĵ�Ч���Ϻã�����������b�Ǽ������������a��û�м������ʱ�������仯���ߣ�D�����
��ѡC��

����Ŀ���ɼ������ӻ�������ɵĻ�����к������������е������֣�K����Cl����NH![]() ��Mg2����Ba2����CO
��Mg2����Ba2����CO![]() ��SO
��SO![]() �����û��������ˮ��ó�����Һ����ȡ����100 mL����Һ�ֱ��������ʵ�顣
�����û��������ˮ��ó�����Һ����ȡ����100 mL����Һ�ֱ��������ʵ�顣
ʵ����� | ʵ������ | ʵ���� |
1 | ����AgNO3��Һ | �а�ɫ�������� |
2 | ��������NaOH��Һ������ | �ռ�������1.12 L(��״����) |
3 | ��������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�������ϡ���ᣬȻ����ˡ�ϴ�ӡ�������� | ��һ�γ�������Ϊ6.27 g���ڶ��γ�������Ϊ2.33 g |
��ش��������⣺
��1������ʵ��1��Cl���Ƿ���ڵ��ж���________(�һ�����ڡ���һ�������ڡ�����ȷ����)������ʵ��1��3�ж�ԭ�������һ�������ڵ�������________��
��2����ȷ��100 mL��Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
�����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
_______ | ___________ |
______ | _______________ |
��3��K���Ƿ���ڣ�________(����ڡ������ڡ�)���жϵ�������____________________��