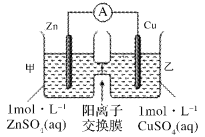
��Ŀ����
����Ŀ������������(FexNy)�ڴż�¼�����������Ź㷺��Ӧ��ǰ����ijFexNy���Ʊ���������������ͪ���Ҵ����롣
��1��Fe3����̬��������Ų�ʽΪ___��
��2����ͪ(CH3CCH3O)������̼ԭ�ӹ�����ӻ�������__��1mol��ͪ�����к�����������ĿΪ___��
��3��C��H��O����Ԫ�صĵ縺����С�����˳��Ϊ____��
��4���Ҵ��ķе���ڱ�ͪ��������Ϊ______��
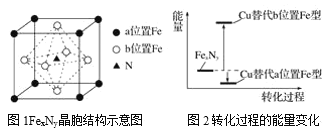
��5��ijFexNy�ľ�����ͼ1��ʾ��Cu������ȫ����þ�����aλ��Fe����bλ��Fe���γ�Cu����Ͳ���Fe(x��n)CunNy��FexNyת��Ϊ����Cu����Ͳ���������仯��ͼ2��ʾ�����и��ȶ���Cu����Ͳ���Ļ�ѧʽΪ____��
1-�������һ����Ҫ���л��ϳ��м��壬�е�Ϊ71�棬�ܶ�Ϊ1.36g��cm��3��ʵ�����Ʊ�����1��������Ҫ�������£�����1��������A�м��������ӡ�12g��������20 mLˮ����ˮ��ȴ�»�������28mLŨH2SO4����ȴ�����£������¼���24gNaBr��
����2����ͼ��ʾ�ʵ��װ�ã��������ȣ�ֱ������״�����Ϊֹ��
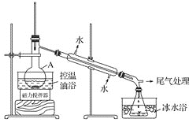
����3�������Һת���Һ©�����ֳ��л��ࡣ
����4�����ֳ����л���ת���Һ©����������12mLH2O��12mL 5% Na2CO3��Һ��12 mL H2Oϴ�ӣ���Һ���ôֲ�Ʒ����һ���ᴿ��1����顣
��1������A��������___�����������ӵ�Ŀ���ǽ����___��
��2����Ӧʱ���ɵ���Ҫ�л���������2������____��
��3������2���������ƿ�ڼ���������ˮ�����ڱ�ˮԡ�е�Ŀ����_____��
��4������2���軺������ʹ��Ӧ������ƽ�Ƚ��У�Ŀ����___��
��5������4����5%Na2CO3��Һϴ���л���IJ��������Һ©����С�ļ���12 mL5%Na2CO3��Һ����___�����ã���Һ��
���𰸡�[Ar]3d5��1s22s22p63s23p63d5 sp2��sp3 9NA H<C<O �Ҵ����Ӽ������� Fe3CuN ������ƿ ��ֹ���� ��ϩ�������� ����1�����Ļӷ� ����HBr�ӷ� ����Һ©���¿�������б�������ų�����
��������
��1������ԭ������Ϊ26��λ�ڵ����������壬![]() ���������Ϊ23�����̬��������Ų�ʽΪ
���������Ϊ23�����̬��������Ų�ʽΪ![]() ��
��![]() ��
��
��2����ͪ�ļ���̼ԭ�ӵ��ӻ���ʽΪ![]() �ӻ����ʻ���̼ԭ�ӵ��ӻ���ʽΪ
�ӻ����ʻ���̼ԭ�ӵ��ӻ���ʽΪ![]() �ӻ���1mol��ͪ�к���6molC-H����2molC-C����1molC=O����������Ϊ�Ҽ���̼��˫���к���1mol�Ҽ�������1mol��ͪ�к���9mol�Ҽ���
�ӻ���1mol��ͪ�к���6molC-H����2molC-C����1molC=O����������Ϊ�Ҽ���̼��˫���к���1mol�Ҽ�������1mol��ͪ�к���9mol�Ҽ���
��3���縺����ԭ���ڷ����������ɼ����ӵ�������ͬ����Ԫ�صĵ縺����ԭ��������������������Ե縺��![]() ������Ϊ�ڼ�����̼Ԫ���Ը����ϼۣ���������������ǿ�����Ե縺��
������Ϊ�ڼ�����̼Ԫ���Ը����ϼۣ���������������ǿ�����Ե縺��![]() ���ʵ縺��
���ʵ縺��![]() ��
��
��4����ͪ���Ӽ�ֻ�з��»��������Ҵ����ڷ��Ӽ������ʹ��е����ߡ�
��5���ɾ���ʾ��ͼ��֪��һ��![]() �����У�������ԭ�ӵ���ĿΪ
�����У�������ԭ�ӵ���ĿΪ![]() ����ԭ�ӵ���ĿΪ1������x=4��y=1����ͼ-2��֪��Cu����þ�����aλ��Fe����ʹ�������ͣ�Cu����þ�����bλ��Fe����ʹ�������ߣ����ȶ���Cu����Ͳ���ΪCu��ȫ����þ�����aλ��Fe����������ͭԭ����ĿΪ
����ԭ�ӵ���ĿΪ1������x=4��y=1����ͼ-2��֪��Cu����þ�����aλ��Fe����ʹ�������ͣ�Cu����þ�����bλ��Fe����ʹ�������ߣ����ȶ���Cu����Ͳ���ΪCu��ȫ����þ�����aλ��Fe����������ͭԭ����ĿΪ![]() ��������ԭ����ĿΪ3���������ȶ���Cu����Ͳ��ﻯѧʽΪ
��������ԭ����ĿΪ3���������ȶ���Cu����Ͳ��ﻯѧʽΪ![]() ��
��
��1������A��������ƿ����������ڴ�������������������ת�ﵽ�����Ŀ�ģ�Ҳ�ܹ�����������г䵱��ʯ�����ã���ֹҺ�屩�С�
��2�����ȹ����У��������ᷢ����������ˮ���ɱ�ϩ����Ӽ���ˮ���������ѡ�
��3������2�е�������¶Ƚϸߣ�����ʹ�ñ�ˮԡ���£����ɵ�1-������ӷ���ʹ�ò��ʽ��͡�
��4������1������е���Ҫ����Ϊ��������HBr�������ȹ�������ʹ�ò���HBr�ӷ����������ȣ��ܹ�������һ���⡣
��5���ֳ����л����лẬ��δ��Ӧ�������ӣ�����![]() ��ˮϴ�Ӻ�����
��ˮϴ�Ӻ�����![]() ���壬��Ҫ����Һ©���¿�������б�������ų����壬Ȼ���÷�Һ��
���壬��Ҫ����Һ©���¿�������б�������ų����壬Ȼ���÷�Һ��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���밴Ҫ��ش��������⣺
(1)�����������Ǵ�����Ⱦ��֮һ���û���̿��һ����̼��ԭ��������ɷ�ֹ������Ⱦ����֪��2C(s)+O2(g)=2CO(g)��H=��22lkJ/mol,
C(s)+O2(g)=CO2(g)��H=��393.5 kJ/mol,
N2(g)+O2(g)=2NO(g)��H=+181 kJ/mol,
��2CO(g)+2NO(g)![]() N2(g)+2CO2(g)��H=__kJ/mol��
N2(g)+2CO2(g)��H=__kJ/mol��
���д�ʩ�ܹ�����˷�Ӧ��NO��ת���ʵ���___(����ĸ���)
a.������������� b.�����¶� c.����CO��Ũ�� d.����NO��Ũ��
(2)���ݻ�Ϊ2L���ܱ������м������̿(����)��NO��������ӦC(s)+2NO(g)![]() N2(g)+CO2(g)��H=��574.5kJ/mol��NO��N2�����ʵ����仯���±���ʾ��
N2(g)+CO2(g)��H=��574.5kJ/mol��NO��N2�����ʵ����仯���±���ʾ��
���ʵ���/mol | T1/�� | T2/�� | |||||
0 | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | |
NO | 2.0 | 1.20 | 0.70 | 0.70 | 0.50 | 0.40 | 0.40 |
N2 | 0 | 0.40 | 0.65 | 0.65 | 0.75 | 0.80 | 0.80 |
��0~5min�ڣ���NO��ʾ�ĸ÷�Ӧ���ʦ�(NO)=__________���������µ�ƽ�ⳣ��K=___________(����2λС��)��
�ڵ�15min���¶ȵ�����T2�����ݱ仯���ϱ���ʾ����T1___________T2(�>������<����=��)��
(3)�ڻ�ѧ�����в���K2CrO4Ϊָʾ������AgNO3����Һ�ζ���Һ��Cl��������Ag+��CrO42������ש��ɫ������ָʾ����ζ��յ㡣����Һ��Cl��ǡ�ó�����ȫ(Ũ�ȵ���1.0��10��6mol��L��1)ʱ����Һ��c(Ag+)Ϊ__mol��L��1����ʱ��Һ��c(CrO42��)����____mol��L��1��(��֪Ksp(Ag2CrO4)=2.0��10��12��Ksp(AgCl)=2.0��10��10)��