��Ŀ����
þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���Ҫ�������£�
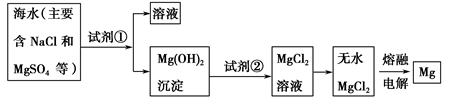
(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ��________��ҪʹMgSO4��ȫת��Ϊ�����������Լ�����ӦΪ________________��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����_____________��
(3)�Լ���ѡ��________��д���䷴Ӧ�����ӷ���ʽ_______��
(4)��ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ________��

(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ��________��ҪʹMgSO4��ȫת��Ϊ�����������Լ�����ӦΪ________________��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����_____________��
(3)�Լ���ѡ��________��д���䷴Ӧ�����ӷ���ʽ_______��
(4)��ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ________��
(1)ʯ���顡������(2)���ˡ�(3)���ᡡMg(OH)2��2H��=Mg2����2H2O��(4)MgCl2(����) Mg��Cl2��
Mg��Cl2��
 Mg��Cl2��
Mg��Cl2��(1)��ҵ�ϳ��������۵�ʯ����ʹ��ˮ�е�MgSO4ת��ΪMg(OH)2��ΪʹMgSO4��ȫת��ΪMg(OH)2��Ӧ�������ʯ���顣
(2)����ʯ�������Mg(OH)2��ͨ�����˽�Mg(OH)2���������
(3)�������ܽ�Mg(OH)2����Ӧ�����ӷ���ʽΪMg(OH)2��2H��=Mg2����2H2O
(4)��ҵ�ϵ������MgCl2��ȡMg����Ӧ�Ļ�ѧ����ʽΪMgCl2(����) Mg��Cl2��
Mg��Cl2��
(2)����ʯ�������Mg(OH)2��ͨ�����˽�Mg(OH)2���������
(3)�������ܽ�Mg(OH)2����Ӧ�����ӷ���ʽΪMg(OH)2��2H��=Mg2����2H2O
(4)��ҵ�ϵ������MgCl2��ȡMg����Ӧ�Ļ�ѧ����ʽΪMgCl2(����)
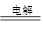 Mg��Cl2��
Mg��Cl2��
��ϰ��ϵ�д�
�����Ŀ

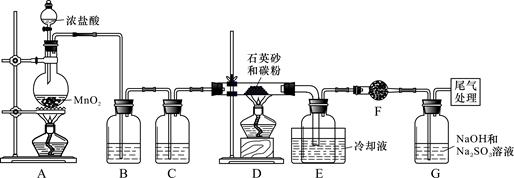
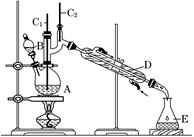
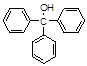 )��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���ϳ�������ͼ1��ʾ��װ����ͼ2��ʾ��
)��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���ϳ�������ͼ1��ʾ��װ����ͼ2��ʾ��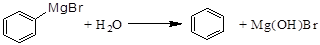 ����ʽ�廯þ����
����ʽ�廯þ����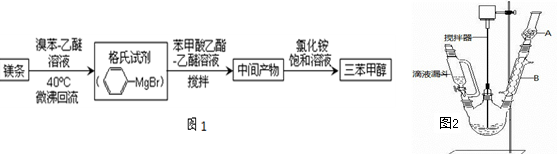
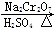 CH3CH2CH2CHO
CH3CH2CH2CHO