��Ŀ����
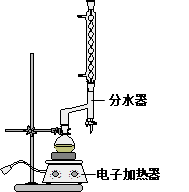
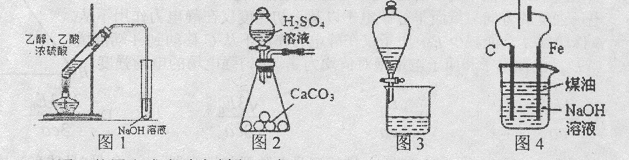
�����״�(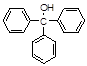 )��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���ϳ�������ͼ1��ʾ��װ����ͼ2��ʾ��
)��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���ϳ�������ͼ1��ʾ��װ����ͼ2��ʾ��
��֪���������Լ�����ˮ�⣬
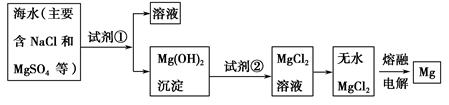
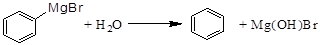 ����ʽ�廯þ����
����ʽ�廯þ����
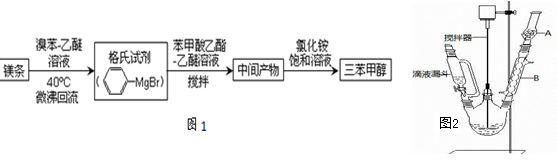
����������ʵ������������£�
���������״��ķ�������260�����������л���һ�㶼�й̶��۵㡣
��ش��������⣺
��1��ͼ2�в�������B�����ƣ� ��װ����ˮCaCl2������A�������� ��
��2��ͼ2�еμ�Һ��δ����ͨ��Һ©�����õ�Һ©���������� ����ȡ�����Լ�ʱҪ�����У����Բ��� ����ʽ�����ȣ��ŵ��� ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д���¿հף�
���У��ٲ���Ϊ�� ��ϴ��Һ���ѡ�ã� (��ѡ����ѡ����
A��ˮ B������ C���Ҵ� D����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ�� ��
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ���������������ƣ��������Ʋ��ᷴӦ������ַ�Ӧ��������������Ϊ100.80ml��״��������Ʒ�������״���������Ϊ ��������λ��Ч���֣���
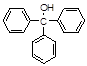 )��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���ϳ�������ͼ1��ʾ��װ����ͼ2��ʾ��
)��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���ϳ�������ͼ1��ʾ��װ����ͼ2��ʾ����֪���������Լ�����ˮ�⣬
 ����ʽ�廯þ����
����ʽ�廯þ����
����������ʵ������������£�
| ���� | �۵� | �е� | �ܽ��� |
| �����״� | 164.2�� | 380�� | ������ˮ�������Ҵ������ѵ��л��ܼ� |
| ���� | -116.3�� | 34.6�� | ����ˮ�������Ҵ��������л��ܼ� |
| �屽 | -30.7��C | 156.2��C | ������ˮ�������Ҵ������ѵȶ����л��ܼ� |
| ���������� | -34.6��C | 212.6��C | ������ˮ |
| Mg(OH)Br | ������Ϊ���� | ������ˮ�������ڴ����ѵ��л��ܼ� | |
���������״��ķ�������260�����������л���һ�㶼�й̶��۵㡣
��ش��������⣺
��1��ͼ2�в�������B�����ƣ� ��װ����ˮCaCl2������A�������� ��
��2��ͼ2�еμ�Һ��δ����ͨ��Һ©�����õ�Һ©���������� ����ȡ�����Լ�ʱҪ�����У����Բ��� ����ʽ�����ȣ��ŵ��� ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д���¿հף�
���У��ٲ���Ϊ�� ��ϴ��Һ���ѡ�ã� (��ѡ����ѡ����
A��ˮ B������ C���Ҵ� D����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ�� ��
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ���������������ƣ��������Ʋ��ᷴӦ������ַ�Ӧ��������������Ϊ100.80ml��״��������Ʒ�������״���������Ϊ ��������λ��Ч���֣���
��1�������ܣ�1�֣�����ֹ������ˮ��������װ�ã�ʹ�����Լ�ˮ�� ��1�֣���
ƽ��ѹǿ������©�����Լ����£�2�֣���ˮԡ ��1�֣� ���Ⱦ��ȣ�
�¶����ڿ��ƣ�1�֣���
��3����������2�֣���A��2�֣���ȡ�������һ��ϴ��Һ���Թ��У��μ�AgNO3��Һ�����������ɣ�����ϴ�Ӹɾ� ���μӷ�̪��Һ��ϴ��Һδ��ɫ���ȣ�����2�֣�
��4��90% ��2�֣�
ƽ��ѹǿ������©�����Լ����£�2�֣���ˮԡ ��1�֣� ���Ⱦ��ȣ�
�¶����ڿ��ƣ�1�֣���
��3����������2�֣���A��2�֣���ȡ�������һ��ϴ��Һ���Թ��У��μ�AgNO3��Һ�����������ɣ�����ϴ�Ӹɾ� ���μӷ�̪��Һ��ϴ��Һδ��ɫ���ȣ�����2�֣�
��4��90% ��2�֣�
�����������1����ͼ2�ɵã���������B�������ܣ���Ϊ�����Լ�����ˮ�⣬��ˮ�Ȼ��������Ը��������Ҫ�Ƿ�ֹ������ˮ��������װ�ã� ��ֹ�����Լ�ˮ�⣬���������״��IJ������ͣ�
��2����Һ©����������Ľṹ��ʹ����ƿ�ڵ�ѹǿ��©���ڵ�ѹǿ��ȣ�ʹ����Һ����˳����������ƿ�У�����ˮ�ķе�Ϊ100�棬ˮԡ���Կ��Ƽ��ȵ��¶Ȳ�����100�棬�ҿ���ʹ��Ӧ�������� ���¶����ڿ��ƣ�
��3�����ݱ��и����������������ݣ������״������ѡ��屽�������������ǻ���Һ�����������ɳɷֵķе����ϴ���˴ֲ�Ʒ����ķ������������������������������Ŀ���dz�ȥ���ѡ��屽�����������������ʣ����ڼ�ʽ�廯þ������ˮ�������ڴ����ѵ��л��ܼ���������ڵ�Ŀ���dz�ȥ��ʽ�廯þ�����������״�������ˮ�������Ҵ������ѵ��л��ܼ������ϴ��Һ���ѡ��ˮ������ѡ�����ѡ��Ҵ��������л���Һ����ֹ���������ѡ��Ҵ��������µ����ʣ������ˮ��������ˮ�Ȼ��Ƶȸ������ȥ���ٴ����ɵõ������������״������ںϳ�����ͼ�м����Ȼ�隣�����Һ����������Ҫ�ɷ��Ǽ�ʽ�廯þ�� ���������������������ӵ����ʣ������������������������ӻ�笠����ӣ����������ӵ����ʿ������ʵ�鷽����������Ƿ�ϴ�Ӹɾ�����ȡ���һ��ϴ��Һ���Թ��У��μ�AgNO3��Һ�����ް�ɫ�������ɣ����Ѿ�ϴ�Ӹɾ���
��4���������������=100.80mL=0.1008L����״��������Ħ�����Ϊ22.4L��mol��1��n=V/Vm�������������ʵ���=0.1008L��22.4L��mol��1�����������״�ֻ����1���ǻ�������2mol�ǻ���2molNa�����û���Ӧ������1molH2����1mol�����״�������Na��Ӧ���ų�0.5mol H2���������״������ʵ�����������2�����������״������ʵ���Ϊ0.1008L��22.4L��mol��1��2�����������״��ķ���ʽΪC19H16O����Է�������Ϊ260��m=noM�����Ʒ�������״�������Ϊ0.1008L��22.4L��mol��1��2��260g��mol��1=2.34g�����ڲ�Ʒ������Ϊ2.60g�����Ʒ�������״�����������Ϊ2.34g��2.60g��100%=90%��

��ϰ��ϵ�д�
 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ

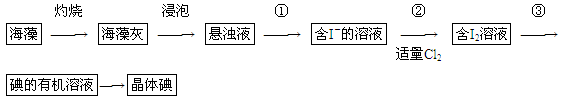 ��1��ʵ������۵�������________��������Ҫ��������Ϊ________��
��1��ʵ������۵�������________��������Ҫ��������Ϊ________��