��Ŀ����
 [��ѧ-���ʽṹ������]
[��ѧ-���ʽṹ������]�������ڹ���Ԫ��Fe��Ti����C��H��N��O�γɶ��ֻ����
��1����H��C��N��O����Ԫ�صĵ縺����С�����˳��Ϊ
��������������ȷ����
A����ΪHCHO��ˮ���Ӽ����γ����������CH2O������ˮ
B��HCHO��CO2�����е�����ԭ�Ӿ�����sp2�ӻ�
C��C6H6�����к���6���Ҽ���1����м���C2H2�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
�����ᣨHOCN����һ����״���ӣ����������ᣨHNCO����Ϊͬ���칹�壬������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ����д������Ľṹʽ
��2��Feԭ�ӻ�������Χ�н϶���������Ŀչ������һЩ���ӻ������γ�����
����Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ������
���������������[Fe��CN��6]4-�����
a�����ۼ� b���Ǽ��Լ� c����λ�� d���Ҽ� e���м�
��д��һ����CN_��Ϊ�ȵ�����ĵ��ʷ���ʽ
�����Ȼ���������Ϊ���壬�۵�282�棬�е�315�棬��300������������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ����ľ�������Ϊ
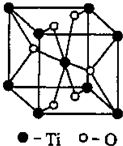
��3����Ti�������ڱ���λ��
��Ti��һ��������X���侧���ṹ����ͼ��ʾ����X�Ļ�ѧʽΪ
��������1����ͬ����Ԫ�ش�����Ԫ�صĵ縺�������ǽ�����Խǿ�縺��Խ��
��A����ȩ��ˮ���Ӽ����γ������
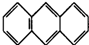
B��������̼������Cԭ��Ϊsp�ӻ���
C������̼̼������6���Ҽ���ÿ��̼�������һ���Ҽ���
D��ԭ�Ӿ�����۷е���ڷ��Ӿ��壻
�۸���ԭ������������ȷ��ԭ���γɵĹ��ۼ���Ŀ���ݴ˽��
��2�����γ������Ӿ߱�������Ϊ������ԭ�Ӿ��пչ����������й¶Ե��Ӷԣ�
���������������[Fe��CN��6]4-��Fe2+��CN-�γ���λ����CN-�д���C��N��������������1���Ҽ�2���м���
CN-��Cԭ����1����λ�������Nԭ���滻�ɵ�CN-�ȵ�����ĵ��ʣ�
������Ŀ��Ϣ��֪���Ȼ������۷е�ͣ��������������Ȼ���Ϊ���Ӿ��壻
��3���ٸ���Tiԭ�Ӻ�������Ų�ʽ���
�ڸ��ݾ�̯�����㾧����Ti��Oԭ�Ӹ�����ȷ�������ﻯѧʽ��TiO2��BaCO3��Ӧ����M��CO2�����������غ㶨�ɿ�֪MΪBaTiO3��
��A����ȩ��ˮ���Ӽ����γ������
B��������̼������Cԭ��Ϊsp�ӻ���
C������̼̼������6���Ҽ���ÿ��̼�������һ���Ҽ���
D��ԭ�Ӿ�����۷е���ڷ��Ӿ��壻
�۸���ԭ������������ȷ��ԭ���γɵĹ��ۼ���Ŀ���ݴ˽��
��2�����γ������Ӿ߱�������Ϊ������ԭ�Ӿ��пչ����������й¶Ե��Ӷԣ�
���������������[Fe��CN��6]4-��Fe2+��CN-�γ���λ����CN-�д���C��N��������������1���Ҽ�2���м���
CN-��Cԭ����1����λ�������Nԭ���滻�ɵ�CN-�ȵ�����ĵ��ʣ�
������Ŀ��Ϣ��֪���Ȼ������۷е�ͣ��������������Ȼ���Ϊ���Ӿ��壻
��3���ٸ���Tiԭ�Ӻ�������Ų�ʽ���
�ڸ��ݾ�̯�����㾧����Ti��Oԭ�Ӹ�����ȷ�������ﻯѧʽ��TiO2��BaCO3��Ӧ����M��CO2�����������غ㶨�ɿ�֪MΪBaTiO3��
����⣺��1����1����ͬ����Ԫ�ش�����Ԫ�صĵ縺�������ǽ�����Խǿ�縺��Խ�ʵ縺�ԣ�H��C��N��O��
�ʴ�Ϊ��H��C��N��O��
��A��A�м�ȩ�к����ǻ�����ˮ�����γ��������A��ȷ��
B��HCHO������Cԭ�Ӳ���sp2�ӻ�����������̼������Cԭ��Ϊsp�ӻ�����B����
C��C2H2��ֱ���ͶԳƽṹ��Ϊ�Ǽ��Է��ӣ�������̼̼������6���Ҽ���ÿ��̼�������һ���Ҽ�����������һ������12������C����
D��������̼�����Ƿ��Ӿ��壬�������辧����ԭ�Ӿ��壬����CO2������۵㡢�е㶼�ȶ������辧��ĵͣ���D��ȷ��
�ʴ�Ϊ��BC��
�����ᣨHOCN����һ����״���ӣ���������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ��̼��4�����ۼ�������3�����ۼ�������2�����ۼ���H��1�����ۼ�������ṹ��ʽΪN��C-O-H��
�ʴ�Ϊ��N��C-O-H��
��2�����γ������Ӿ߱�������Ϊ������ԭ�Ӿ��пչ����������й¶Ե��Ӷԣ�
�ʴ�Ϊ�����й¶Ե��ӣ�
���������������[Fe��CN��6]4-��Fe2+��CN-�γ���λ����CN-�д���C��N������Ϊ���Թ��ۼ�����������1���Ҽ�2���м�������CN-�й��ۼ�����λ�����Ҽ����м���
CN-��Cԭ����1����λ�������Nԭ���滻�ɵ�CN-�ȵ�����ĵ���ΪN2��
�ʴ�Ϊ��b��N2��
������Ŀ��Ϣ��֪���Ȼ������۷е�ͣ������������������ѡ���ͪ���л��ܼ��������Ȼ������ڷ��Ӿ��壬
�ʴ�Ϊ�����Ӿ��壻
��3����Tiԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d24s2����λ�ڵ������ڵڢ�B�壬����d����
�ʴ�Ϊ���������ڵڢ�B�壻d����
��ÿ�������к�����ԭ����Ϊ8��
+1=2����ԭ����Ϊ4��
+2=4���������ﻯѧʽΪTiO2��TiO2��BaCO3��Ӧ����M�������������������ΪCO2�����������غ㶨�ɿ�֪MΪBaTiO3����Ӧ����ʽΪ��TiO2+BaCO3�TBaTiO3+CO2����
�ʴ�Ϊ��TiO2��TiO2+BaCO3�TBaTiO3+CO2����
�ʴ�Ϊ��H��C��N��O��
��A��A�м�ȩ�к����ǻ�����ˮ�����γ��������A��ȷ��
B��HCHO������Cԭ�Ӳ���sp2�ӻ�����������̼������Cԭ��Ϊsp�ӻ�����B����
C��C2H2��ֱ���ͶԳƽṹ��Ϊ�Ǽ��Է��ӣ�������̼̼������6���Ҽ���ÿ��̼�������һ���Ҽ�����������һ������12������C����
D��������̼�����Ƿ��Ӿ��壬�������辧����ԭ�Ӿ��壬����CO2������۵㡢�е㶼�ȶ������辧��ĵͣ���D��ȷ��
�ʴ�Ϊ��BC��
�����ᣨHOCN����һ����״���ӣ���������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ��̼��4�����ۼ�������3�����ۼ�������2�����ۼ���H��1�����ۼ�������ṹ��ʽΪN��C-O-H��
�ʴ�Ϊ��N��C-O-H��
��2�����γ������Ӿ߱�������Ϊ������ԭ�Ӿ��пչ����������й¶Ե��Ӷԣ�
�ʴ�Ϊ�����й¶Ե��ӣ�
���������������[Fe��CN��6]4-��Fe2+��CN-�γ���λ����CN-�д���C��N������Ϊ���Թ��ۼ�����������1���Ҽ�2���м�������CN-�й��ۼ�����λ�����Ҽ����м���
CN-��Cԭ����1����λ�������Nԭ���滻�ɵ�CN-�ȵ�����ĵ���ΪN2��
�ʴ�Ϊ��b��N2��
������Ŀ��Ϣ��֪���Ȼ������۷е�ͣ������������������ѡ���ͪ���л��ܼ��������Ȼ������ڷ��Ӿ��壬
�ʴ�Ϊ�����Ӿ��壻
��3����Tiԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d24s2����λ�ڵ������ڵڢ�B�壬����d����
�ʴ�Ϊ���������ڵڢ�B�壻d����
��ÿ�������к�����ԭ����Ϊ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��TiO2��TiO2+BaCO3�TBaTiO3+CO2����
���������⿼�������ʽṹ�����ʣ���Ŀ�Ƚ��ۺϣ����ض����ʽṹ����֪ʶ�Ŀ��飬�漰�縺�ԡ��ӻ����ۡ���ѧ�������ӽṹ�����ʡ��������������ʡ���������ȣ���Ҫѧ���߱�֪ʶ�Ļ������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

 ��ƽ��ṹ������
��ƽ��ṹ������ ��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�
��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�