��Ŀ����
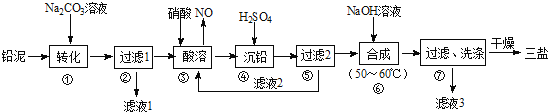
����Ŀ���÷�Ǧ���ص�Ǧ��(��PbSO4��PbO��Pb��)���Ʊ���ϸ������Ʒ3PbO��PbSO4��H2O(����)����Ҫ�Ʊ��������¡�
��ش��������⣺
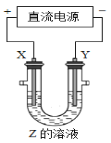
(1)Ǧ�������������й㷺Ӧ�ã��乤��ԭ����Pb+PbO2+2H2SO4![]() 2PbSO4+2H2O����Ǧ���طŵ�ǰ��������������ȣ��ŵ�ʱת����1 mol���ӣ�����������������֮��Ϊ__________��
2PbSO4+2H2O����Ǧ���طŵ�ǰ��������������ȣ��ŵ�ʱת����1 mol���ӣ�����������������֮��Ϊ__________��
(2)����Һ1����Һ3�ϲ���������Ũ�������½ᾧ�����˵Ȳ������ɵõ�һ�ֽᾧˮ����(Mr=322)���仯ѧʽΪ______________________��
(3)���������ʱǦ�����ᷴӦ����Pb(NO3)2��NO����Һ2�����ʵ���Ҫ�ɷ�Ϊ______(�ѧʽ)��
(4)����ϳ����εĻ�ѧ����ʽΪ______________________��
(5)����ߵ�ϴ�Ӳ����У���������Ƿ�ϴ����ȫ�IJ���������__________________��
���𰸡�16 gNaSO4��10H2OHNO34PbSO4+6NaOH![]() 3PbO��PbSO4��H2O��+3Na2SO4+2H2Oȡ�������һ��ϴ�Ӻ����Һ���Թ��У��μ�BaCl2��Һ�����ᣬ��������ɫ�����������δϴ����ȫ����û�а�ɫ�������ɣ��������ϴ����ȫ
3PbO��PbSO4��H2O��+3Na2SO4+2H2Oȡ�������һ��ϴ�Ӻ����Һ���Թ��У��μ�BaCl2��Һ�����ᣬ��������ɫ�����������δϴ����ȫ����û�а�ɫ�������ɣ��������ϴ����ȫ
��������
��Ǧ���м���̼������Һ�� PbSO4ת��Ϊ����PbCO3�����ӷ���ʽΪCO32-+ PbSO4= PbCO3+SO42-��Ȼ����˵õ���Һ��Ϊ��������Һ���������м����������������PbO��Pb��PbCO3�������ᷴӦ����Pb(NO3)2��Pb�����ᷴӦ������NO��Ǧ����������Pb(NO3)2��NO�����ӷ���ʽΪ3Pb+8H++2NO3-=Pb2++2NO��+4H2O��Ȼ������Һ�м�������������PbSO4��������������Һ������Ҫ�ɷ���HNO3,�������м���NaOH��Һ,������Ӧ��4PbSO4+6NaOH![]() 3PbO��PbSO4��H2O��+3Na2SO4+2H2O,����ϴ�Ӹ���õ�3PbO��PbSO4��H2O����Һ���к���NaSO4���Դ˽�������
3PbO��PbSO4��H2O��+3Na2SO4+2H2O,����ϴ�Ӹ���õ�3PbO��PbSO4��H2O����Һ���к���NaSO4���Դ˽�������
(1)Ǧ�����ڳ��ʱ,��������������Ӧ, PbSO4����������PbO2���缫��ӦʽΪPbSO4+2H2O-2e-=PbO2+4H++SO42-����Ǧ���طŵ�ǰ������������������ŵ�ʱ����������PbO2+2e-+SO42-+4H+=PbSO4+2H2O����������Pb-2e-+SO42-=PbSO4��ת����lmol������������������������0.5molPbSO4, ��������������m(PbSO4)-m(PbO2)=0.5��(303-207)=32g��������������m(PbSO4)-m(Pb)= 0.5��(303-207)=48g,������������Ϊ48-32=16g����ˣ�������ȷ������16 g��
(2) ͨ�����Ϸ���֪����Һ������Һ������ɫ���������ᾧ�ɵõ��ĸ���ƷΪNaSO4��10H2O������Է�������Ϊ322����ˣ�������ȷ����: NaSO4��10H2O��
(3) ͨ�����Ϸ���֪�������ӷ�Ӧ����ʽΪ3Pb+8H++2NO3-=Pb2++2NO��+4H2O����Һ2����Ҫ�ɷ���δ��Ӧ��HNO3�������������ȷ����: HNO3��
(4)����Ǧ�����������ڼ��ȵ�50-60������·�Ӧ����3PbO��PbSO4��H2O��Na2SO4������ϳ����εĻ�ѧ����ʽΪ4PbSO4+6NaOH![]() 3PbO��PbSO4��H2O��+3Na2SO4+2H2O����˱�����ǣ�4PbSO4+6NaOH
3PbO��PbSO4��H2O��+3Na2SO4+2H2O����˱�����ǣ�4PbSO4+6NaOH![]() 3PbO��PbSO4��H2O��+3Na2SO4+2H2O��
3PbO��PbSO4��H2O��+3Na2SO4+2H2O��
(5)�ó����������������������������������Ȼ�����Һ���м���������鷽��Ϊȡ�������һ��ϴ�Ӻ����Һ���Թ����������еμ��������ٵμ�BaCl2��Һ������������ɫ�������������ϴ����ȫ����ˣ�������ȷ����: ȡ�������һ��ϴ�Ӻ����Һ���Թ��У��μ�BaCl2��Һ�����ᣬ��������ɫ�����������δϴ����ȫ����û�а�ɫ�������ɣ��������ϴ����ȫ��

����Ŀ����1����ҵ���Դ�ͭΪԭ�ϲ�ȡ����ͼ��ʾ�����Ʊ�����ͭ���壺

���ڲ���a�У�����Ҫͨ��������ˮ����Ŀ����___________________________��
���ڱ���ȥ���Ĺ����У�ΪʹFe3+������ȫ����������Һ�м���CuO��������Һ��pH�������±����ݣ���Һ��pHӦ������____________��Χ��
�������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
Fe3+ Cu2+ | 1.9 4.7 | 3.2 6.7 |
���ü�ˮ�ķ���������ҺpH��ԭ����_______________________________________��
�۽�������Ũ��ʱ��Ҫ�����������Һ��pH=1����Ŀ���ǣ�������ӷ���ʽ˵����
_________________________________________________________________________��
��2����ҵ�ϳ���������ͭ��Һ���ͭ�����ʱ�����ĵ缫��Ӧʽ��______________________��
��3����ͼ��ijС��ͬѧ�������������������ͭ����[ Cu��NO3��2��nH2O ]���ܽ�����ߣ��¶���30��ǰ���Ӧ��ͬ�ľ��壩������˵����ȷ����__________������ĸ����

a��A��ʱ����ҺΪ��������Һ
b��B��ʱ���־�����Թ���
c���������������յõ��ľ�����Cu��NO3��2��3H2O
d������C��ʱ����Һ������30�����£���������Cu��NO3��2��6H2O����
��4��ijЩ���ۻ������H2O��NH3��N2O4�ȣ���Һ̬ʱ�����ĵ����ԣ���Ҫ����Ϊ�����˵��룬�磺2NH3![]() NH
NH![]() +NH
+NH![]() ���ɴ��Ʊ���ˮ����ͭ�ķ���֮һ����Cu��Һ̬N2O4��Ӧ��Һ̬N2O4����õ������������������������18����Һ̬N2O4����ķ���ʽ��______________________________________��Cu��Һ̬N2O4��Ӧ�Ƶ���ˮ����ͭ�Ļ�ѧ����ʽ��______________________________________��
���ɴ��Ʊ���ˮ����ͭ�ķ���֮һ����Cu��Һ̬N2O4��Ӧ��Һ̬N2O4����õ������������������������18����Һ̬N2O4����ķ���ʽ��______________________________________��Cu��Һ̬N2O4��Ӧ�Ƶ���ˮ����ͭ�Ļ�ѧ����ʽ��______________________________________��
����Ŀ������ϩ����Ҫ�Ļ���ԭ�ϣ�������ϩ����ȲΪԭ�Ϻϳɡ���Ӧ����������ʾ��
��Ȳ�ۺϣ�3C2 H2 (g)![]() C6H6 (g) H1
C6H6 (g) H1
�ϳ��ұ���C6H6(g)+C2H4(g)![]() C6H5CH2CH3(g) H2
C6H5CH2CH3(g) H2
�ұ����⣺C6H5CH2CH3(g)![]() C6H5CH=CH2 (g)+H2 (g) ��H3
C6H5CH=CH2 (g)+H2 (g) ��H3
(1)�ұ������Ǻϳɱ���ϩ�Ĺؼ����衣ij�¶��£���2.0 L�����ܱ������г���0.10mol C6H5CH2CH3 (g)������ұ����ⷴӦʱ��(t)��������������ѹǿ(p)�����ݼ��±���
ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
��ѹǿp/100kPa | 4.91 | 5.58 | 6.32 | 7.31 | 8.54 | 9.50 | 9.52 | 9.53 | 9.53 |
�ټ�����¶��µ�ƽ�ⳣ��K= ______�����������С�������λ����
�����в���˵�����¶��·�Ӧ�ﵽƽ��״̬����_____������ĸ���ţ���
a.v(C6H5CH2CH3)=v(H2) b������ϩ�������������
c��ƽ�ⳣ��K���ֲ��� d����������ƽ����Է�����������
(2)�����Ϊ3.0 L�ĺ����ܱ������г���0. 20 mol C6H5CH2CH3(g)�����ұ����ⷴӦ�ﵽƽ��״̬ʱ��ƽ����ϵ��ɣ����ʵ������������¶ȵĹ�ϵ��ͼ��ʾ��

�ٸ÷�Ӧ��H3______0������ڡ������ڡ���С�ڡ�����
�ڸ�ƽ����ϵ��600��ʱ���ұ������ʵ�������Ϊ50%�������������ʵ�������Ϊ_____�����ڴ��¶��¼���ˮ������ϡ�ͼ������ұ���ƽ��ת���ʽ���α仯����������__________��
(3)����ϩ��������KMnO4��Һ��Ϸ�Ӧ���ɱ�����(C6H5COOH)�������£��ͱ�������Һ�м���̼�����ƹ���ʹ��Һ�����ԣ�����Һ��c(C6H5COOH)��c(C6H5COO-)=___________������֪���������Ka=6.4��l0-5��̼���Kal=4.2��l0-7��Ka2=5.6��l0-ll)