��Ŀ����
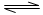
��������������(HF)n��ʽ���ڣ���֪�����������д��������ƽ�⣺
H3F3 3HF (����ӦΪ���ȷ�Ӧ)
3HF (����ӦΪ���ȷ�Ӧ)
H2F2 2HF (����ӦΪ���ȷ�Ӧ)
2HF (����ӦΪ���ȷ�Ӧ)
�����������У�����ȷ����
H3F3
 3HF (����ӦΪ���ȷ�Ӧ)
3HF (����ӦΪ���ȷ�Ӧ)H2F2
 2HF (����ӦΪ���ȷ�Ӧ)
2HF (����ӦΪ���ȷ�Ӧ)�����������У�����ȷ����
| A���������ƽ��Ħ������Ϊ40 g/mol����H3F3�������������Ϊ10% |
| B�����ڶ��¶������ٳ���H3F3����H3F3��HF��Ũ��(mol/L)��ֵ���� |
| C���������ƽ��Ħ������Ϊ42 g/mol����H3F3�����������С��10% |
| D�����ڶ��¶����ٳ���HF����H3F3��HF��Ũ��(mol/L)��ֵ���� |
C
��

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ
 2C��g�� ��2���ӷ�Ӧ�ﵽƽ�⣬��ʱ����0.2mol C��
2C��g�� ��2���ӷ�Ӧ�ﵽƽ�⣬��ʱ����0.2mol C��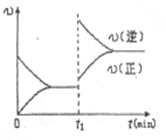
 2NH3����һ���������Ѵﵽƽ��״̬��
2NH3����һ���������Ѵﵽƽ��״̬�� p C(g)����H = a kJ/mol ( a<0)�����ں��¡������´ﵽƽ��ʱA��ת����ΪA1% ���ھ��ȡ����������´ﵽƽ��ʱA��ת����ΪA2%����A1��A2�Ĺ�ϵΪ
p C(g)����H = a kJ/mol ( a<0)�����ں��¡������´ﵽƽ��ʱA��ת����ΪA1% ���ھ��ȡ����������´ﵽƽ��ʱA��ת����ΪA2%����A1��A2�Ĺ�ϵΪ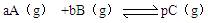 ��H=������Ӧ�����¼���±���
��H=������Ӧ�����¼���±��� L-1
L-1 in
in Z(g)��2W(g)����ƽ��ʱ,A�����Ϊ1.2aL������˵����ȷ����
Z(g)��2W(g)����ƽ��ʱ,A�����Ϊ1.2aL������˵����ȷ����

 xC(g)��2minʱ��Ӧ�ﵽƽ��״̬���¶Ȳ��䣩��ʣ����0.8molB�������C��Ũ��Ϊ0.4mol��L������д���пհף�
xC(g)��2minʱ��Ӧ�ﵽƽ��״̬���¶Ȳ��䣩��ʣ����0.8molB�������C��Ũ��Ϊ0.4mol��L������д���пհף� 2SO3(g)���������������ٱ仯ʱ������˵����ѧ��Ӧ�Ѿ��ﵽƽ��״̬����
2SO3(g)���������������ٱ仯ʱ������˵����ѧ��Ӧ�Ѿ��ﵽƽ��״̬���� nC(g)+2D(g) ���ﵽƽ���A���ʵ���Ũ�ȼ�С1/2����������ƽ��Ħ����������1/8����÷�Ӧ�Ļ�ѧ����ʽ��n��ֵ��
nC(g)+2D(g) ���ﵽƽ���A���ʵ���Ũ�ȼ�С1/2����������ƽ��Ħ����������1/8����÷�Ӧ�Ļ�ѧ����ʽ��n��ֵ��