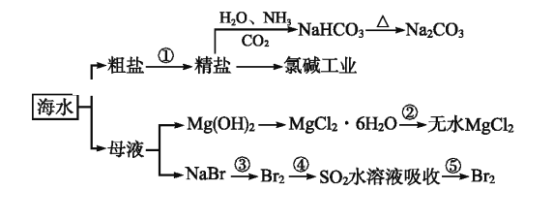
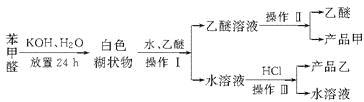
��Ŀ����
����Ŀ��ʵ�����Ʊ����״��ͱ�����Ļ�ѧԭ����
��֪����ȩ�ױ��������������״��ķе�Ϊ205.3 ������������۵�Ϊ121.7 �����е�Ϊ249 �����ܽ��Ϊ0.34 g�����ѵķе�Ϊ34.8 ����������ˮ���Ʊ����״��ͱ��������Ҫ����������ʾ��
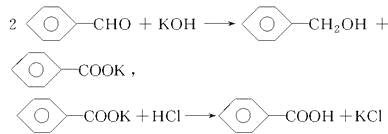
�Ը���������Ϣ���ж�����˵���������(����)
A. ����������ȡ��Һ
B. ������Һ�����ܽ����Ҫ�ɷ��DZ��״�
C. �������������ò�Ʒ���DZ��״�
D. ���������˵õ���Ʒ���DZ������
���𰸡�D
�����������������A���ӹ����Ͽ��������õ�������Һ��ˮ��Һ���������ǻ������ܵ�Һ�壬������ȡ�ͷ�Һ�ķ�������ȷ��B������ȩ���������������ɱ��Ҵ��ͱ�����أ������Ʊ�������ͱ��Ҵ����Ʊ����̣�������Һ�ܽ����Ҫ�ɷ��DZ��״�����ȷ��C�������������˱��״��ķе�Ϊ205.3�������ѵķе�Ϊ34.8���������ܣ����÷е㲻ͬ����������ķ����õ����Ѻͱ��Ҵ�����ȷ��D��������ˮ��Һ�м������ᣬ�������ת��ɱ����ᣬ���������ˮ�����ù��˵ķ������з��룬����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������SO2��CO��NOx��Ⱦ�ǻ�ѧ�������о�����Ҫ���⡣
��.���᳧�����ŷź�SO2��β����Ի����������Σ����
��1����ҵ�Ͽ����÷ϼ�Һ����Ҫ�ɷ�ΪNa2CO3���������᳧β���е�SO2���õ�Na2SO3��Һ���÷�Ӧ�����ӷ���ʽΪ__________________________________��
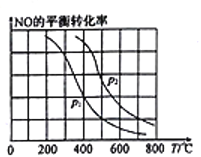
��.�������������Ϊ��Ӧ��2CO(g)+O2(g)![]() 2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��
2CO2(g)�Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ�����,ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��

��2��a��b��c��d �ĵ��У��ﵽƽ��״̬����__________________________________��
��3����֪c��ʱ������O2Ũ��Ϊ0.02 mol/L����50��ʱ���ڦ��������������COת����Ӧ��ƽ�ⳣ��K=____________���ú�x�Ĵ���ʽ��ʾ����
��4�����й���ͼ��˵����ȷ����_____________��
A.COת����Ӧ��ƽ�ⳣ��K(a)
B.�ھ�δ�ﵽƽ��״̬ʱ��ͬ���¦��������������COת�����ʱȦ���Ҫ��
C.b��ʱCO��O2����֮�䷢����Ч��ײ�ļ���������ʵ����������
D.e��ת���ʳ���ͻ���ԭ��������¶����ߺ����ʧȥ����
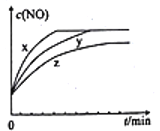
��.ij���ܴ������Դ��������ͳ�β���е�̼��(C)��NOx����ͬ�¶��£���ģ��β�����ɷ����±���ʾ������ͬ������ͨ���ô�����������в��CO2��N2��N2O����NO��������ݽ����ͼ����ʾ��
ģ��β�� | ����(10mol) | ̼�� | ||
NO | O2 | He | ||
���ʵ���(mol) | 0.025 | 0.5 | 9.475 | n |
��5��375��ʱ������ų��������к�0.45 molO2��0.0525 mol CO2����Y�Ļ�ѧʽΪ______________��

��6��ʵ������в���NOģ��NOx����������NO2��ԭ����____________________________��