��Ŀ����
����Ŀ��ʵ���ǻ�ѧ�о���һ����Ҫ�ֶΣ�������ͼ��ʾA��E���������������Ҫ����ա�
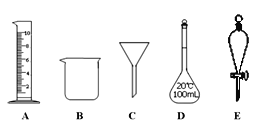
(1)д���������ƣ�C__________��E_________��
(2)����ʵ��������õ�����E����___________������ĸ����
a.����ˮ��CCl4�Ļ����
b.����ˮ�;ƾ��Ļ����
c.����ˮ����ɳ�Ļ����
d.����NaCl��Һ�е�NaCl��ˮ
(3)����A��E��ʹ��ǰ�������Ƿ�©ˮ����_________��
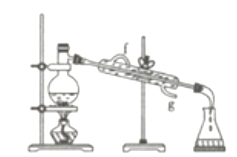
(4)��������ͼװ�÷������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������____________������ǰӦ����Բ����ƿ�м��뼸����ʯ��Ŀ����_________________________�������ܵĽ�ˮ����________������f������g������
���𰸡�����ͨ��©�� ��Һ©�� a DE �¶ȼ� ��ֹ���� g
��������
(1)����������״д�����ƣ�
(2)����GΪ��Һ©���������ڷ�Һ��
(3)����������ʹ��Ŀ���ж��ĸ���Ҫ����Ƿ�©Һ��
(4)����������Һ������Ҫ���¶ȼƲ�������¶ȣ�Ҫ��ֹ��������ķ�������������ԭ�����¡�
(1)����������״C��©����EΪ��Һ©����
(2)����GΪ��Һ©���������ڷ��뻥�����ݵ�����Һ�����ʣ�
a.ˮ��CC14�ǻ������ݵ�����Һ�����ʣ����÷�Һ©�����룬a��ȷ��
b.�ƾ���ˮ���ܵ�Һ�����ʣ������÷�Һ���룬b����
c.��ɳ��������ˮ�Ĺ������ʣ�Ӧ�ù��˷������룬c����
d.ʳ��ˮ�е�����NaCl���ܼ�ˮ�������ܼ������룬d����
�ʺ���ѡ����a��
(3)A����Ͳ��������ȡһ�������Һ�����ʣ�����Ҫ����Ƿ�©Һ��A����
B.���ձ����������ȡ�������Һ��ϡ�͵ȣ�����Ҫ����Ƿ�©Һ��B����
C.��װ����©�����������˻���С��������ת����Һ������Ҫ����Ƿ�©Һ��C����
D.Ϊ����ƿ��ֻ���ڳ�����ʹ�ã���������һ�����һ��Ũ�ȵ���Һ����ʹ��ʱҪ����Һҡ�ȣ����ʹ��ǰҪ����Ƿ�©ˮ��D��ȷ��
E.Ϊ��Һ©�������ڷ��뻥�����ݵ�����Һ�����ʣ�Ϊ��ֹ�����Һ�������ٻ�ϣ�ʹ��ǰҪ����Ƿ�©ˮ��E��ȷ��
�ʺ���ѡ����DE��
(4)���Ȼ�̼�;ƾ��ǻ��ܵ�Һ��������ڶ��߷е����ϴɲ����������룬����ͼʾ��֪����ȱ�ٵ��������¶ȼƣ�Һ���������ʱ��Ϊ��ֹҺ�屩�У�Ҫ�������Ƭ�������Ȳ�������ֽ��£�Ϊ��������Ч����ʹ�������г�����ˮ��Ҫ��������ԭ����������ˮ�����g��

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�����Ŀ��q����ƽ�ⳣ���Ǻ���������ʵ���̶ȵ�������֪���±�����(25��):
��ѧʽ | ����ƽ�ⳣ�� |
HCN | K=4.9��10-10 |
CH3COOH | K=1.8��10-5 |
H2CO3 | K1=4.4��l0-7��K2=4.7��10-11 |
��1�� 25��ʱ����Ũ�ȵ�������Һ(a.NaCN��Һ��b.Na2CO3 ��Һ��c.CH3COONa ��Һ)��pH�ɴ�С��˳��Ϊ__________________��(��д���)
��2��25��ʱ����NaCN��Һ��ͨ������CO2�� ��������Ӧ�Ļ�ѧ����ʽΪ__________________��
��3������Ũ��Ϊ0.02 mol/L��HCN��0.01mol/L NaOH�������Ϻ��c(Na+ )>c(CN-)�����й�ϵ��ȷ����__________________��
A. c(H+)>c(OH-) B.c(H+)-)
C. c(H+)+c(HCN)= C(OH-) D. c(HCN)+c(CN-)=0.01mol/L
��4�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±�:
ʵ���� | HA���ʵ���Ũ��(mol/L) | NaOH���ʵ���Ũ��(mol/L) | �����Һ��pH |
a | 0.1 | 0.1 | pH=9 |
b | c | 0.2 | pH=7 |
��ش�:
�ٴ�a�����������HA ��ǿ�ỹ������___________________��
��b�����������C____0.2 (ѡ������������С��������������)�������Һ������Ũ��c(A-)___c(Na+)(ѡ������������С��������������)��
��a��ʵ�����û����Һ����ˮ�������c (OH-)=___mol/L��д���û����Һ��������ʽ�ľ�ȷ���(���������Ƽ���)��c (Na+)-c(A-)=____mol/L��
��5����֪��100��ʱ��ˮ�����ӻ�Ϊ1��10-12����ʱ��pH=11 ��NaOH��ҺV1 L��pH=2��H2SO4��ҺV2L���Ȼ�Ϻ������û����Һ��pH=10����V1:V2Ϊ_________��
��6��HAΪ���ᣬ�������£�������ˮϡ��0.01 mol/L HA��Һʱ�����гʼ�С���Ƶ���____��
A.c(H+)/c(A-) B.c(HA)/c(A-)
C.��Һ��c(H+)��c(OH-)�ij˻� D.��Һ��c(A-)��c(HA)��ֵ E.ˮ�ĵ���̶�