��Ŀ����
����Ŀ��A��B��C��D��E��F��ԭ��������������Ķ���������Ԫ�أ�A��E��Ԫ�����ڱ��е����λ����ͼ��ʾ��A����Ԫ�����γ�������ɫ���壬C�ǵؿ��к�������Ԫ�أ�D�ǵؿ��к������Ľ���Ԫ�ء�

��1��C��Ԫ�����ڱ��е�λ��Ϊ________��������ӵĽṹʾ��ͼΪ________________��
��2��AE2�ķ���ʽΪ__________��
��3��C��D��E��F�����Ӱ뾶�ɴ�С��˳����___________(�����ӷ���)��
��4��ʵ������ȡF�ĵ�����������ӷ���ʽΪ________________________��
��5�������ӹ�ҵ�У�B�ļ���̬�⻯���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ___________________��
���𰸡��ڶ����ڵڢ�A�� 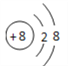 CS2 S2>Cl>O2>Al3+ MnO2+4H++2Cl
CS2 S2>Cl>O2>Al3+ MnO2+4H++2Cl![]() Mn2++Cl2��+2H2O 2NH3��H2O+3H2O2��N2��+8H2O
Mn2++Cl2��+2H2O 2NH3��H2O+3H2O2��N2��+8H2O
��������
������������Ϣ��֪��A��B��C��D��E��F��ԭ��������������Ķ���������Ԫ�أ�A������Ԫ���γ�������ɫ���壬��AΪC(̼)Ԫ�أ�C�ǵؿ��к�������Ԫ�أ�CΪOԪ�أ�D�ǵؿ��к������Ľ���Ԫ�أ�DΪAlԪ�أ�BΪNԪ�أ���ͼ�и�����A��E��Ԫ�����ڱ��е����λ�ã���E��S��FΪCl��
(1)CΪ��Ԫ�أ������ڱ��е�λ��Ϊ�ڶ����ڵڢ�A�壬������ӽṹʾ��ͼΪ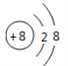 ��(2)AΪC(̼)��EΪS�����AE2�ķ���ʽΪCS2��(3)������ͬ�ĵ��Ӳ�ṹ�����ӣ��˵����Խ��ԭ�Ӻ˶Ժ�����ӵ���������Խǿ�����Ӱ뾶ԽС�����в�ͬ���Ӳ�ṹ�����ӣ����Ӳ���Խ�࣬���Ӱ뾶Խ����˿ɵ����Ӱ뾶��S2��Cl��O2��Al3+��(4)ʵ������ȡ���������ö������̺�Ũ���ᷴӦ�������ӷ���ʽΪMnO2+4H++2Cl
��(2)AΪC(̼)��EΪS�����AE2�ķ���ʽΪCS2��(3)������ͬ�ĵ��Ӳ�ṹ�����ӣ��˵����Խ��ԭ�Ӻ˶Ժ�����ӵ���������Խǿ�����Ӱ뾶ԽС�����в�ͬ���Ӳ�ṹ�����ӣ����Ӳ���Խ�࣬���Ӱ뾶Խ����˿ɵ����Ӱ뾶��S2��Cl��O2��Al3+��(4)ʵ������ȡ���������ö������̺�Ũ���ᷴӦ�������ӷ���ʽΪMnO2+4H++2Cl![]() Mn2++Cl2��+2H2O��(5)BΪN�������̬�⻯��ΪNH3����ˮ��ҺΪ��ˮ����ˮ��H2O2��Ӧ�Ļ�ѧ����ʽΪ2NH3��H2O+3H2O2��N2��+8H2O��
Mn2++Cl2��+2H2O��(5)BΪN�������̬�⻯��ΪNH3����ˮ��ҺΪ��ˮ����ˮ��H2O2��Ӧ�Ļ�ѧ����ʽΪ2NH3��H2O+3H2O2��N2��+8H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�