��Ŀ����
6��������з���ʽ����1��CH3CH2CHO+Cu��OH��2��CH3CH2CHO+Cu��OH��2$\stackrel{��}{��}$H2O+2Ag��+3NH3+CH3CH2COOH��
��2��OHC-CHO+Ag��NH3��2OH����������OHC-CHO+4Ag��NH3��2OH��������$\stackrel{��}{��}$H4NOOCCOONH4+6NH3+2H2O��
��3��
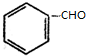 +H2����������
+H2���������� +H2��������$\stackrel{����}{��}$
+H2��������$\stackrel{����}{��}$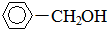 ��
��
���� ��1����ȩ�����Ƶ�������ͭ��Ӧ�����ɱ����������ͭ��ˮ��
��2���Ҷ�ȩ��������Һ��Ӧ�����Ҷ���͵�������
��3�����б�����ȩ�������ʿ��Ժ�����֮�䷢���ӳɷ�Ӧ��
��� �⣺��1����ȩ�����Ƶ�������ͭ��Ӧ�����ɱ����������ͭ��ˮ����ӦΪ��CH3CH2CHO+Cu��OH��2$\stackrel{��}{��}$H2O+2Ag��+3NH3+CH3CH2COOH��
�ʴ�Ϊ��CH3CH2CHO+Cu��OH��2$\stackrel{��}{��}$H2O+2Ag��+3NH3+CH3CH2COOH��
��2���Ҷ�ȩ��������Һ��Ӧ�����Ҷ���͵�������OHC-CHO+4Ag��NH3��2OH��������$\stackrel{��}{��}$H4NOOCCOONH4+6NH3+2H2O��
�ʴ�Ϊ��OHC-CHO+4Ag��NH3��2OH��������$\stackrel{��}{��}$H4NOOCCOONH4+6NH3+2H2O��
��3�� ��H2���������ӳɿ��Եõ����״�����
��H2���������ӳɿ��Եõ����״����� +H2��������$\stackrel{����}{��}$
+H2��������$\stackrel{����}{��}$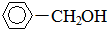 ��
��
�ʴ�Ϊ�� +H2��������$\stackrel{����}{��}$
+H2��������$\stackrel{����}{��}$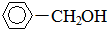 ��
��
���� ���⿼��ѧ���л���ѧ��Ӧ����ʽ����д֪ʶ��ע�����ʵ����ʵ������ǹؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
17�����и���������ǿ������Һ���ܴ���������ǣ�������
| A�� | Na+��K+��H+��SO42- | B�� | Ba2+��Na+��NO3-��Cl- | ||
| C�� | Na+��K+��S2-��CO32- | D�� | Ba2+��Cu2+��NO3-��Cl- |
14������ʵ�顰�����������롰���ۡ��Ķ�Ӧ��ϵ��ȷ���ǣ�������
| ���������� | ���� | |
| A | �����½���Ƭ����Ũ�����У������Ա仯 | Al��Ũ�����Ӧ |
| B | ��0.1mol•L-1 Na2CO3��Һ�У��μ�2�η�̪����Һ��dz��ɫ���ȣ���ɫ���� | ����ˮ�������ȷ�Ӧ |
| C | ��FeCl3��Һ�м���������ͭ�ۣ���ַ�Ӧ��ֹ��ȡ�ϲ���Һ�μӼ���KSCN��Һ����Һ�����ɫ | �����ԣ�Cu 2+��Fe3+ |
| D | ��2mL 1mol•L-1 NaOH��Һ���ȼ���3��1mol•L-1 MgCl2��Һ���ټ���3��1mol•L-1FeCl3��Һ�������ɰ�ɫ��ɺ��ɫ | Mg��OH��2��������ת��ΪFe��OH��3���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
1����֪���Ȼ�����S2Cl2���ĽṹʽΪCl-S-S-Cl��������ˮ��Ӧ2S2Cl2+2H2O=4HCl+SO2��+3S�����Ը÷�Ӧ��˵����ȷ���ǣ�������
| A�� | S2Cl2����������������ԭ�� | |
| B�� | H2O����ԭ�� | |
| C�� | ÿ����1molSO2ת��4mol���� | |
| D�� | ���������뻹ԭ�������ʵ�����Ϊ3��1 |
11������ȩ���Ҵ�����������������ɵĻ����0.1mol����ȫȼ�պ�����������ͨ��Ũ���ᣬȻ����ͨ��ʢ�м�ʯ�ҵĸ���ܣ����Ũ���������������5.4�ˣ���ԭ���������ȩ���Ҵ������������ʵ���֮��Ϊ��������
| A�� | 2��3��1 | B�� | 1��2��3 | C�� | 4��3��2 | D�� | 3��2��1 |
18���������2��3��ϵ�N2��CO2 112.8g�ڱ�״�������Ϊ��������
| A�� | 22.4L | B�� | 44.8L | C�� | 67.2L | D�� | 89.6L |