��Ŀ����
16������ʽΪC2H6O�Ļ�����A�����������ʣ�A+Na�������������ݣ���1������������Ϣ���Ըû�������������ж���AD��
A��һ������-OH��������B�������ԡ�����C��AΪ���ᡡ����D��AΪ�Ҵ�
��2��A����������Ʒ�Ӧʱ�����ѵĻ�ѧ��ΪO-H���������Ԫ�أ���ͬ����������Ϊȩʱ�����ѵĻ�ѧ��ΪO-H�����ǻ����ӵ�̼ԭ���ϵ�C-H����
��3����A���������Ϊ75%��ˮ��Һ��������ҽ����������
��4��A+CH3COOH$��_{��}^{ŨH_{2}SO_{4}}$��������ζ�IJ����ѧ��Ӧ����ʽΪCH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
��5������������е�������16O��A�е�������18O��������ŨH2SO4�����·�����Ӧ������һ��ʱ������к���18O��������B��
A.1�� B.2�� C.3�� D.4��
��6��A���ɺ�����[��C6H10O5��n]��ũ��Ʒ�����ס�С������Ⱦ����͡�������ã���д���ɵ�����A�Ļ�ѧ����ʽ����C6H10O5��n+nH2O$��_{��}^{����}$nC6H12O6��C6H12O6$\stackrel{�ƻ�ø}{��}$2CH3CH2OH+2CO2����
���� ��1������ʽΪC2H6O�Ļ�����A����Na��Ӧ�������壬��A����-OH����AΪCH3CH2OH��
��2���Ҵ����Ʒ�Ӧ�����Ҵ���������������������������ȩ������л���Ľṹ�жϻ�ѧ���Ķ��ѣ�
��3��75%�ľƾ�ˮ��Һ��������ҽ����������
��4���������Ҵ���Ũ���������·�����Ӧ��������������
��5��������Ӧ�������ṩ�ǻ������ṩ�ǻ�������ˮ���������ֽ����������������Ӧ���ڿ��淴Ӧ��
��6������ˮ�����������ǣ��������ھƻ�ø�����·ֽ����ɾƾ��������̼��
��� �⣺��1������ʽΪC2H6O�Ļ�����A����Na��Ӧ�������壬��A����-OH����AΪCH3CH2OH���ʴ�Ϊ��AD��
��2���Ҵ����Ʒ�Ӧ�����Ҵ���������������O-H����������Ϊȩʱ����O-H�����ǻ����ӵ�̼ԭ���ϵ�C-H����
�ʴ�Ϊ��O-H����O-H�����ǻ����ӵ�̼ԭ���ϵ�C-H����
��3��75%�ľƾ�ˮ��Һ��������ҽ�����������ʴ�Ϊ��ҽ����������
��4���������Ҵ���Ũ���������·�����Ӧ���������������÷�ӦΪ��CH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOC2H5+H2O���ʴ�Ϊ��CH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOC2H5+H2O��
��5��������Ӧ�������ṩ�ǻ������ṩ�ǻ�������ˮ���������ֽ����������������Ӧ��CH3CH218OH+CH3C16O16OH$?_{��}^{Ũ����}$CH3C16O18OC2H5+H216O�������к���18O�������У�CH3CH218OH��CH3C16O18OC2H5��
��ѡ��B��
��6������ˮ�����������ǣ��������ھƻ�ø�����·ֽ����ɾƾ��������̼����Ӧ����ʽΪ����C6H10O5��n+nH2O$��_{��}^{����}$nC6H12O6��C6H12O6$\stackrel{�ƻ�ø}{��}$2CH3CH2OH+2CO2����
�ʴ�Ϊ����C6H10O5��n+nH2O$��_{��}^{����}$nC6H12O6��C6H12O6$\stackrel{�ƻ�ø}{��}$2CH3CH2OH+2CO2����
���� ���⿼���Ҵ��Ľṹ�����ʡ���;���Ʊ��Լ��������ʵȣ��Ƚϻ����������Ҵ�������������ǹؼ���

| A�� | NaF��CH3COONa��NaCN | B�� | NaCN��CH3COOHNa��NaF | ||
| C�� | CH3COOHNa��NaF��NaCN | D�� | NaF��NaCN��CH3COONa |
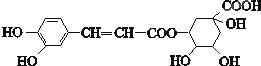
| A�� | ����ʽΪC16H18O9 | |
| B�� | 0.1mol��ԭ������뺬0.4molNaOH��Һ��Ӧ | |
| C�� | �ܷ���ȡ������ԭ����������ȥ��Ӧ | |
| D�� | 0.1mol��ԭ������뺬0.6molBr2��Ũ��ˮ��Ӧ |
| ��ѧ��Ӧ | ƽ�ⳣ�� | �¶ȣ��棩 | |
| 500 | 800 | ||
| ��2H2��g��+CO��g��?CH3OH��g�� | K1 | 2.5 | 0.15 |
| ��H2��g��+CO2��g��?H2O��g��+CO��g�� | K2 | 1.0 | 2.5 |
| ��3H2��g��+CO2��g��?CH3OH��g��+H2O��g�� | K3 | ||
��2��ij�¶��·�Ӧ����H2��ƽ��ת���ʣ�a������ϵ��ѹǿ��p���Ĺ�ϵ��ͼ����ʾ����ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��=K��B�����������������=������
��3���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3=K1•K2����K1��K2��ʾ����500��ʱ��÷�Ӧ����ijʱ��ʱ��H2��g����CO2��g����CH3OH��g����H2O��g����Ũ�ȣ�mol•L-1���ֱ�Ϊ0.8��0.1��0.3��0.15�����ʱv����v�����������=����������
��4����3L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c��CO��-t����Ӧʱ�䣩�仯���ߢ���ͼ����ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ�
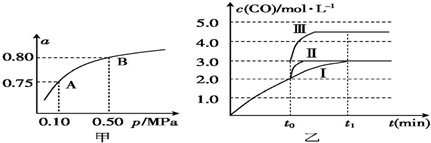
�����ߢ��Ϊ���ߢ�ʱ���ı�������Ǽ������
�����ߢ��Ϊ���ߢ�ʱ���ı�����ǽ��������������ѹ����2L��
A��CuO��CO2��SO2��H2O B�� Cl2��Na��Fe��Cu
C��HCl��HNO3��H2SO4��H2O D��Na2CO3��KCl��NaOH��AgNO3
| A�� | B�� | C�� | D�� | |
| ����� | �ǽ��������� | �������� | �� | �� |
| �����ڸ��������� | CuO | Cl2 | H2O | NaOH |
| A�� | ֻ�Т� | B�� | ֻ�Т٢ڢ� | C�� | ֻ�Тڢ� | D�� | ȫ�� |
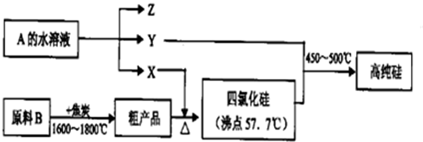
 ������һ����;�൱�㷺�ĸ߷��Ӿۺ��ij�о�С����Ƶ�����ϩΪԭ�Ϻϳ�PVA��������ͼ��
������һ����;�൱�㷺�ĸ߷��Ӿۺ��ij�о�С����Ƶ�����ϩΪԭ�Ϻϳ�PVA��������ͼ��
 ��
��