��Ŀ����
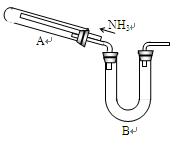
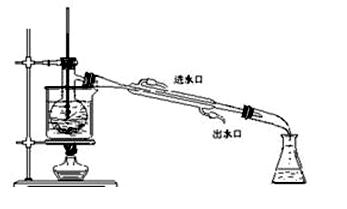
ij�о���ѧϰС���������²�����̽��NH3�Ļ�ԭ�ԣ�����ʵ��װ������ͼ��
����1��NH3��ǿ��ԭ�����ܽ�ijЩ���������ﻹԭΪ�������ʻ�ͼ�̬��������磺
2NH3 + 3CuO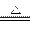 3Cu + N2 +3H2O
3Cu + N2 +3H2O
����2��Cu+��������Һ�в��ȶ����ɷ�������������ԭ��Ӧ����Cu2O����ɫ��������Cu2+��Cu��
Cu2O + 2H+ ="=" Cu2+ + Cu +H2O
��ش��������⣺
(1)Ϊ֤��NH3��ԭCuO�ķ�Ӧ����ˮ���ɣ�B��Ӧ������Լ��� ��
(2)���۲쵽 ��������������A�еķ�Ӧ�Ѿ���ɣ�
(3)��С�����������Ϊ1�U4��ϡ�������Լ������鷴Ӧ�Ƿ���Cu2O�������ɡ�����98%��Ũ��������1�U4��ϡ���ᣬ����IJ����������˽�ͷ�ι���� ��
(4)��֤����ԭ�����к���Cu2O�IJ����������� ��
(5)��д��A������Cu2O�Ļ�ѧ����ʽ ��
(6)���ö����ķ����ⶨ�÷�Ӧ�Ƿ�����Cu2O��������ȷ�ķ�����
��
����1��NH3��ǿ��ԭ�����ܽ�ijЩ���������ﻹԭΪ�������ʻ�ͼ�̬��������磺
2NH3 + 3CuO
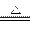 3Cu + N2 +3H2O
3Cu + N2 +3H2O����2��Cu+��������Һ�в��ȶ����ɷ�������������ԭ��Ӧ����Cu2O����ɫ��������Cu2+��Cu��
Cu2O + 2H+ ="=" Cu2+ + Cu +H2O

��ش��������⣺
(1)Ϊ֤��NH3��ԭCuO�ķ�Ӧ����ˮ���ɣ�B��Ӧ������Լ��� ��
(2)���۲쵽 ��������������A�еķ�Ӧ�Ѿ���ɣ�
(3)��С�����������Ϊ1�U4��ϡ�������Լ������鷴Ӧ�Ƿ���Cu2O�������ɡ�����98%��Ũ��������1�U4��ϡ���ᣬ����IJ����������˽�ͷ�ι���� ��
(4)��֤����ԭ�����к���Cu2O�IJ����������� ��
(5)��д��A������Cu2O�Ļ�ѧ����ʽ ��
(6)���ö����ķ����ⶨ�÷�Ӧ�Ƿ�����Cu2O��������ȷ�ķ�����
��
1����ˮ����ͭ
��2��A�еĺ�ɫ������ȫ��ɺ�ɫ������2�֣�
��3���ձ� ��Ͳ ������ ��3�֣�
��4������������Һ����Һ����ɫ ��3�֣�
��5��2NH3+6CuO 3Cu2O +N2+3H2O��3�֣�
3Cu2O +N2+3H2O��3�֣�
��6��������Ӧǰ��װ��A�й����������2�֣�
��2��A�еĺ�ɫ������ȫ��ɺ�ɫ������2�֣�
��3���ձ� ��Ͳ ������ ��3�֣�
��4������������Һ����Һ����ɫ ��3�֣�
��5��2NH3+6CuO
 3Cu2O +N2+3H2O��3�֣�
3Cu2O +N2+3H2O��3�֣���6��������Ӧǰ��װ��A�й����������2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 mo
mo L��NO2�����ʵ���Ϊ moL��
L��NO2�����ʵ���Ϊ moL�� ��Ӧ���ɵ�����������������Һ��ȫ��ת��ΪNaNO3,������Ҫ��������Ϊ30%��˫��ˮ g��
��Ӧ���ɵ�����������������Һ��ȫ��ת��ΪNaNO3,������Ҫ��������Ϊ30%��˫��ˮ g�� ��
��