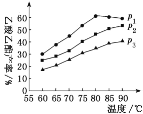
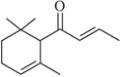
��Ŀ����
����Ŀ����ͼ��ij�о���ѧϰС�������ȡ������������Ϊ��Ӧ������ض���Ӧ��װ��
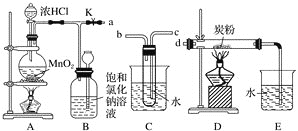
(1)Ҫ��Cװ�ý���B��D֮�䣬��ȷ�Ľӷ��ǣ�a��________��________�� d ��
(2)ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ�����K����Cl2��������װ�ã��ٵ�ȼD���ľƾ��ơ�Cl2ͨ��Cװ�ú����D��Dװ����ʢ��̿�ۣ�����������ԭ��Ӧ������CO2��HCl(g)��������Ӧ�Ļ�ѧ����ʽΪ__________________________________________��Ϊ��ʹCװ�÷��Ӹ��õ����ã������ձ��м���Ũ���ᣬ����Ũ�����������_________________________________________________________________��
(3) D����Ӧ��Ϻرյ��ɼ�K����ȥ�����ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е�������_________________________________________________________��B��������______________________________________________________��
(4)A��B��C��D��Eװ������һ����Ҫ�Ľ���˵����Ҫ�Ľ������ɲ��ڷ����л����Ľ����װ��ͼ��
__________________________________________
���𰸡�c b 2Cl2��C��2H2O![]() 4HCl��CO2 Ũ��������ˮ�ܷų��������ȣ��������Թ��в���ˮ���� ƿ�ϲ�����Ϊ����ɫ��ƿ��Һ���½�������©����Һ������ ��������Cl2������Cl2�Ի��������Ⱦ ��Ӧ��������к���HCl��δ��Ӧ��Cl2��ˮ���������������ܲE��Ӧ��NaOH��Һ�����ӷ�����װ��
4HCl��CO2 Ũ��������ˮ�ܷų��������ȣ��������Թ��в���ˮ���� ƿ�ϲ�����Ϊ����ɫ��ƿ��Һ���½�������©����Һ������ ��������Cl2������Cl2�Ի��������Ⱦ ��Ӧ��������к���HCl��δ��Ӧ��Cl2��ˮ���������������ܲE��Ӧ��NaOH��Һ�����ӷ�����װ��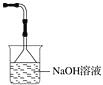
��������
(1)Ҫʹ������C����D�������ܵ������dz����̳���
Cl2ͨ��Cװ�ú����D��Dװ����ʢ��̿�ۣ�����������ԭ��Ӧ������CO2��HCl(g)������Ԫ���غ㣬�μӷ�Ӧ��������C��Cl2��H2O��Cװ�õ��������ṩˮ������
(3)�ر�K����������װ��B��
(4) ˮ���������������ܲE��Ӧ��NaOH��Һ�����ӷ�����װ�ã�
(1)Ҫʹ������C����D�������ܵ������dz����̳�����ȷ�Ľӷ��ǣ�a��c��b�� d ��
(2)Cl2ͨ��Cװ�ú����D��Dװ����ʢ��̿�ۣ�����������ԭ��Ӧ������CO2��HCl(g)������Ԫ���غ㣬�μӷ�Ӧ��������C��Cl2��H2O����Ӧ����ʽ��2Cl2��C��2H2O![]() 4HCl��CO2��Cװ�õ��������ṩˮ������Ũ��������ˮ�ܷų��������ȣ��������Թ��в���ˮ������
4HCl��CO2��Cװ�õ��������ṩˮ������Ũ��������ˮ�ܷų��������ȣ��������Թ��в���ˮ������
(3)�ر�K����������װ��B��ƿ�ϲ�����Ϊ����ɫ��ƿ��Һ���½�������©����Һ��������װ��B����������������Cl2������Cl2�Ի��������Ⱦ��
(4) ��Ӧ��������к���HCl��δ��Ӧ��Cl2��ˮ���������������ܲE��Ӧ��NaOH��Һ�����ӷ�����װ�ã� ��
��

 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�