题目内容
【题目】室温下,用相同浓度的NaOH溶液,分别滴定浓度均为0.1mol.L-l的三种酸(HA、HB和HD)溶液,滴定曲线如图所示,下列判断错误的是

A.三种酸的电离常数关系:KHA>KHB>KHD
B.滴定至P点时,溶液中:c(B-)>c(Na+)>c(HB)>c(H+)>c(OH-)
C.pH=7时,三种溶液中:c(A-)=c(B-)=c(D-)
D.当中和百分数达100%时,将三种溶液混合后:c(HA) +c (HB) +c (HD) =c (OH-) -c (H+)
【答案】C
【解析】
试题分析:A.根据图像可知0.1mol·L-1的三种酸(HA、HB和HD)溶液的起始pH值HA最小,酸性最强,HD的pH最大,酸性最弱,酸性越强,电离平衡常数越大,三种酸的电离常数关系:KHA>KHB>KHD,A正确;B.滴定至P点时溶质为等物质的量浓度的HB和NaB,溶液显酸性,HB的电离为主,但电离程度较小,因此c(B-)>c(Na+)>c(HB)>c(H+)>c(OH-) ,B正确;C.pH=7时,三种溶液中阴离子的水解程度不同,加入的氢氧化钠的体积不同,三种离子浓度分别和钠离子浓度相等,但三种溶液中钠离子浓度不等,C错误;D.此为混合溶液的质子守恒关系式,c(HA)+c(HB)+c(HD)=c(OH-)-c(H+),D正确;答案选C。

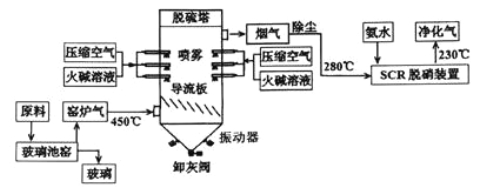
【题目】重铬酸钾(K2Cr2O7)是工业生产和实验室的重要氧化剂,工业上常用铬铁矿(主要成分为FeO·Cr2O3)为原料生产重铬酸钾。实验室模拟工业法用铬铁矿制K2Cr2O7的主要工艺如下图所示。反应器中涉及的主要反应是:6 FeO·Cr2O3 + 24NaOH + 7KClO3 = 12Na2CrO4 + 3Fe2O3 + 7KCl + 12H2O

试回答下列问题:
(1)在反应器①中,有Na2CrO4生成,同时Fe2O3转变为NaFeO2,杂质SiO2、Al2O3与纯碱反应的原理相同均转变为可溶性盐。写出氧化铝与碳酸钠反应的化学方程式:______________________。
(2)操作⑤中加酸酸化后CrO42-转化为Cr2O72-,写出转化的离子方程式:_________________。
(3)步骤③中测定pH值的操作为:________________。
(4)某同学设计的检验上述工艺流程④的滤渣中Fe、Al、Si元素成分的探究实验如下,请帮助完成该实验,并填写表中空格:
操作步骤 | 实验现象 | 结论 |
①取滤渣样少量于试管中,加足量稀HCl,搅拌,静置。 | 试管中仍有固体物质 | 固体为_____________ |
②操作①后,过滤;在滤液中加入过量的____________。 | 有红褐色沉淀产生 | 沉淀为Fe (OH)3 |
③将操作②后的红褐色沉淀滤去,在滤液中通入足量CO2。 | _____________ | 生成物为Al (OH)3; |
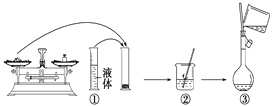
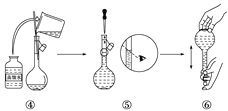
(5)称取重铬酸钾(其式量为294)试样2.5000 9配成250 mL溶液,用移液管取出25. 00 mL于碘量瓶中,加入10 mL 2 mol/L H2SO4和足量碘化钾(铬的还原产物为Cr3+),放置于暗处5 min,然后加入100 mL水,加入3 mL淀粉指示剂,用0.1200 mol/L Na2S2O3标准溶液滴定(I2+2S2O32-=2I-+S4O62-)。
①配制溶液时所需的玻璃仪器除烧杯、玻璃棒外,还需________和________________。
②若实验中共用去Na2S2O3标准溶液40. 00 mL,则所得产品中重铬酸钾的纯度为(设整个过程中其他杂质不参与反应)____________。(保留2位小数)