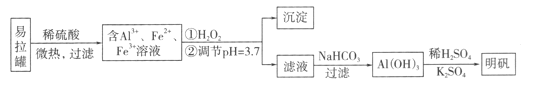
��Ŀ����
����Ŀ��˫������[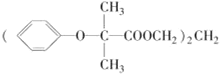 ]�����ڽ���ѪҺ�еĵ��̴��������ʺϳ���·��ͼ��ʾ��
]�����ڽ���ѪҺ�еĵ��̴��������ʺϳ���·��ͼ��ʾ��
��֪����.RCH2COOH![]()
![]()
![]()
![]() ��
��
��.RCH=CH2![]() RCH2CH2Br��
RCH2CH2Br��
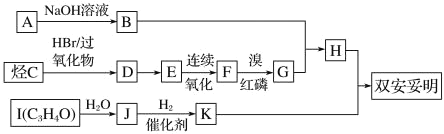
��.C ���ܶ���ͬ��ͬѹ�� H2 �ܶȵ� 28 ������֧����һ������I�ܷ���������Ӧ�� 1 molI(C3H4O)���� 2 mol H2 �����ӳɷ�Ӧ��K �Ľṹ���жԳ��ԡ��Իش�
(1)A�Ľṹ��ʽΪ__________��
(2)��Ӧ D��E �Ļ�ѧ����ʽΪ________�� ��Ӧ������_______��
(3)C ������Ϊ______��
(4)�� F ��Ϊͬ���칹�壬�����������л�����_____�֡����к˴Ź��������� 3 ��壬�����֮��Ϊ 6��1��1 �Ľṹ��ʽΪ_____��
���𰸡�![]() (CH3)2CHCH2Br��NaOH
(CH3)2CHCH2Br��NaOH![]() (CH3)2CHCH2OH��NaBr ȡ����Ӧ 2-����ϩ 4
(CH3)2CHCH2OH��NaBr ȡ����Ӧ 2-����ϩ 4 ![]()
��������
��˫�������Ľṹ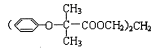 ��֪���ϳ�˫������������ΪHOCH2CH2CH2OH��
��֪���ϳ�˫������������ΪHOCH2CH2CH2OH��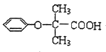 ��I�ķ���ʽΪC3H4O������ϵ�з�Ӧ����K������Cԭ����Ŀ��֪��KΪHOCH2CH2CH2OH��I�ܷ���������Ӧ�������к���-CHO����1molI����2mol���������ӳɷ�Ӧ��˵�������л�����C=C˫������IΪCH2=CH-CHO��JΪHOCH2CH2CHO��HΪ
��I�ķ���ʽΪC3H4O������ϵ�з�Ӧ����K������Cԭ����Ŀ��֪��KΪHOCH2CH2CH2OH��I�ܷ���������Ӧ�������к���-CHO����1molI����2mol���������ӳɷ�Ӧ��˵�������л�����C=C˫������IΪCH2=CH-CHO��JΪHOCH2CH2CHO��HΪ ��B��G��Ӧ����
��B��G��Ӧ���� ���ɷ�Ӧ��ϢI��֪���ϳ�H������Ϊ�����ơ� (CH3)2CBr COOH��C���ܶ���ͬ��ͬѹ��H2�ܶȵ�28����˵��C����Է�������Ϊ56����
���ɷ�Ӧ��ϢI��֪���ϳ�H������Ϊ�����ơ� (CH3)2CBr COOH��C���ܶ���ͬ��ͬѹ��H2�ܶȵ�28����˵��C����Է�������Ϊ56����![]() =4��C�ķ���ʽΪC4H8����֧����һ����������ϵ��ת������G������Cԭ����Ŀ��֪��GΪ (CH3)2CBrCOOH����BΪ�����ƣ�A���������Ʒ�Ӧ����B����AΪ����
=4��C�ķ���ʽΪC4H8����֧����һ����������ϵ��ת������G������Cԭ����Ŀ��֪��GΪ (CH3)2CBrCOOH����BΪ�����ƣ�A���������Ʒ�Ӧ����B����AΪ����![]() ��F����/��������������G�����ݷ�Ӧ��ϢI����֪FΪ(CH3)2CHCOOH��E������������F����EΪ(CH3)2CHCH2OH��Dת������E��DΪ(CH3)2CHCH2Br���ɷ�Ӧ��ϢII��֪��CΪ(CH3)2C=CH2���ݴ˷������
��F����/��������������G�����ݷ�Ӧ��ϢI����֪FΪ(CH3)2CHCOOH��E������������F����EΪ(CH3)2CHCH2OH��Dת������E��DΪ(CH3)2CHCH2Br���ɷ�Ӧ��ϢII��֪��CΪ(CH3)2C=CH2���ݴ˷������
(1)ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)��ӦD��E�Ļ�ѧ����ʽΪ��(CH3)2CHCH2Br+NaOH![]() (CH3)2CHCH2OH+NaBr������±������ˮ�ⷴӦ��Ҳ��ȡ����Ӧ���ʴ�Ϊ��(CH3)2CHCH2Br+NaOH
(CH3)2CHCH2OH+NaBr������±������ˮ�ⷴӦ��Ҳ��ȡ����Ӧ���ʴ�Ϊ��(CH3)2CHCH2Br+NaOH![]() (CH3)2CHCH2OH+NaBr��ȡ����Ӧ��
(CH3)2CHCH2OH+NaBr��ȡ����Ӧ��
(3)CΪ(CH3)2C=CH2������Ϊ��2-��-1-��ϩ���ʴ�Ϊ��2-��-1-��ϩ��
(4)FΪ(CH3)2CHCOOH����F��Ϊͬ���칹�����������������У��������������������������������������������4�֣����к˴Ź��������������壬�����֮��Ϊ6��1��1�Ľṹ��ʽΪ��HCOOCHCH3)2���ʴ�Ϊ��4��HCOOCH(CH3)2��

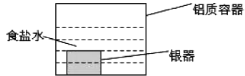
����Ŀ����ʯī�缫������е��ʵ��
ʵ��һ | ʵ��� | |
װ�� |
|
|
���� | a��d����ֽ����;b�����,�ֲ���ɫ;c�������Ա仯 | ����ʯī�缫���������ݲ���;n�������ݲ������� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ�������ǣ� ��
A. a��d����2H2O+2e-=H2��+2OH- B. b����2Cl--2e-=Cl2��
C. c�������˷�Ӧ��Fe-2e-=Fe2+ D. ����ʵ��һ��ԭ��,ʵ�����m��������ͭ
����Ŀ��CO2�Ļ����������ǿ�ѧ���о����ȵ���⡣������CH4��CO2�Ʊ��ϳ���(CO��H2)�������Ʊ��״��������ѡ���̼ϩ����ȼ�ϲ�Ʒ��
I.��ѧ������Ʊ����ϳ�������Ӧ���̷�������
��Ӧ�٣�CH4(g)C(ads) +2H2(g) (����Ӧ)
��Ӧ�ڣ�C(ads) + CO2(g)2CO(g) (�췴Ӧ)
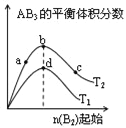
������Ӧ��C(ads)Ϊ�����Ի���̿����Ӧ���̵������仯��ͼ��
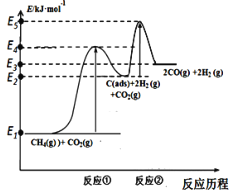
(1)CH4��CO2�Ʊ��ϳ������Ȼ�ѧ����ʽΪ____________���÷�Ӧ�ڸ����¿��Է�������е�ԭ����_________�������仯ͼ�У�E5+E1___E4+E2(����>������<������=��)��
II.�������ϳ������ϳ��괼����ˮ�Ƶö����ѡ�
��ӦΪ��2CH3OH(g)CH3OCH3 (g) + H2O(g) ��H
���������ϣ���һ����Χ�ڣ�������Ӧ��ѧƽ�ⳣ��������ѧ�¶ȴ������¹�ϵ��lnKc=-2.205+![]() �������ʷ���Ϊ��v��=k��c2(CH3OH)��v��=k��c(CH3OCH3)c(H2O)��k����K��Ϊ���ʳ�������Ӱ������ֻ���¶ȡ�
�������ʷ���Ϊ��v��=k��c2(CH3OH)��v��=k��c(CH3OCH3)c(H2O)��k����K��Ϊ���ʳ�������Ӱ������ֻ���¶ȡ�
(2)��Ӧ�ﵽƽ��������¶ȣ�k������ı���________ k������ı���(����>������<������= ��)��
(3)ij�¶��£�Kc=200�����ܱ������м���һ����CH3OH����Ӧ��ijʱ�̲�ø���ֵ����ʵ������£�
���� | CH3OH | CH3OCH3 | H2O |
���ʵ���/mol | 0.4 | 0.4 | 0.4 |
��ʱ�����淴Ӧ���ʵĴ�С��v�� ____v��(����>������<������=��)��
(4)500K�£����ܱ������м���һ�����״�CH3OH����Ӧ����ƽ��״̬ʱ����ϵ��CH3OCH3(g)�����ʵ�������Ϊ_____(�����)
A.![]() B.
B.![]() C.
C.![]() D.��ȷ��
D.��ȷ��
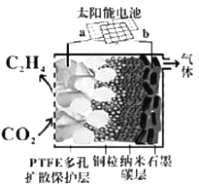
��.�ϳɵ�̼ϩ��
(5)ǿ���Ե����ԭCO2�Ʊ���ϩ�о�ȡ��ͻ�ƽ�չ��ԭ����ͼ��ʾ��b���ӵ���̫���ܵ�ص�_______��(��֪PTFE�����˱���KCl��Һ)����д�������ĵ缫��Ӧʽ______��