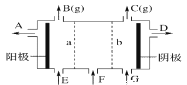
��Ŀ����
����Ŀ��Ԫ��X��Q��Y��Z��M��R��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��Mԭ�ӵ����������������������֮��Ϊ3��4��R����Z����X�����Ӱ뾶��С��������XR������Ϊ���壬Q��Y�����ڱ��е�λ�����ڡ���ش��������⡣
(1)Mԭ�ӵļ۵����Ų�ͼ��__________��Q�ij����⻯������幹����_________________��
(2)д��X��Y��R��ԭ�Ӹ���֮��1��1��1�γɵĻ�����Ľṹʽ:_______________��
(3)X��Y�ɷֱ��γ�10���Ӻ�18���ӵķ��ӣ�д����18���ӷ���ת����10���ӷ��ӵĻ�ѧ����ʽ:_________��
(4)д������R��һ�ֹ�ҵ��;:_______________��
(5)ͼ��ʾ������Ԫ���е�ij����Ԫ����ɵ����������һ�������µ��ܱ������г�ַ�Ӧǰ���ת����ϵ��д����ת�����̵Ļ�ѧ����ʽ:_______��
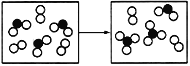
(6)��X��Y��Z��M����Ԫ�ؿ��γ�һ�����ӻ�����A����֪A���������ᷴӦ������������������Һ��Ӧ�����ܺ���ˮ��Ӧ��д��A����ˮ��Ӧ�����ӷ���ʽ:_____��
(7)��ѧ����Ϊ����QX5�������ʣ���Ԥ������ˮ���ҷ�Ӧ�ų����壬����ˮ��Һ�������ԣ�д���÷�Ӧ�Ļ�ѧ����ʽ:______________��
���𰸡�![]() ������ H-O-Cl 2H2O2
������ H-O-Cl 2H2O2![]() 2H2O��O2�� ��Ư��(����Ư��Һ����������ˮ�����Ⱥ����𰸾���) 2SO2+O2
2H2O��O2�� ��Ư��(����Ư��Һ����������ˮ�����Ⱥ����𰸾���) 2SO2+O2![]() 2SO3 HSO3-+H2O+Cl2=SO42-+2Cl-+3H+ NH5��H2O=H2��+NH3��H2O �� NH5+H2O=H2��+NH3��+H2O �� NH5=H2��+NH3��
2SO3 HSO3-+H2O+Cl2=SO42-+2Cl-+3H+ NH5��H2O=H2��+NH3��H2O �� NH5+H2O=H2��+NH3��+H2O �� NH5=H2��+NH3��
��������
Ԫ��X��Q��Y��Z��M��R��Ϊ����������Ԫ�أ���ԭ��������������YԪ��ԭ������������������������֮��Ϊ3��4������������ֻ��Ϊ6����ԭ������Ϊ8��YΪOԪ�أ�Mԭ�ӵ����������������������֮��Ϊ3��4������������Ҳֻ��Ϊ6����MΪSԪ�أ����R-��Z+��X+����������ɿ�֪��RԪ��λ�ڵڢ�A�壬Z��Xλ�ڵ�IA�壬Rԭ��������������RΪCl��������XR�ڳ�����Ϊ���壬��X��HԪ�أ�Z��ԭ����������O����ZΪNa��Q��Y�����ڱ��е�λ�����ڣ���QΪNԪ�ء�
��������������֪��X��H��Q��N��Y��O��Z��Na��M��S��R��ClԪ�ء�
(1)MΪSԪ�أ�ԭ�Ӻ������������Ӳ㣬���������Ų�ʽ��3s23p4���۵����Ų�ͼΪ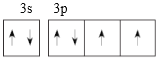 ��Q��NԪ�أ��䳣���⻯��NH3�����幹��Ϊ�����Σ�
��Q��NԪ�أ��䳣���⻯��NH3�����幹��Ϊ�����Σ�
(2)X��Y��R�ֱ���H��O��Cl������Ԫ�ذ�ԭ�Ӹ���֮��1��1��1�γɵĻ�����HClO�ĽṹʽΪH-O-Cl��
(3)HԪ����OԪ���γɵ�10���ӡ�18���ӵķ��ӷֱ�ΪH2O��H2O2��H2O2���ȶ������Ȼᷢ���ֽⷴӦ������H2O��O2�����18���ӷ���ת����10���ӷ��ӵĻ�ѧ����ʽ��2H2O2![]() 2H2O��O2����
2H2O��O2����
(4)R�ĵ���Ϊ�������ڹ�ҵ�Ͽ�����Ư�ۣ���Ư��Һ������ˮ�����ȣ�
(5)��ͼ��֪����ת������ΪSO2��O2��Ӧ����SO3����Ӧ��ѧ����ʽΪ��2SO2+O2![]() 2SO3��
2SO3��
(6)��H��O��Na��S����Ԫ����ɵ�һ�����ӻ�����A��A���������ᷴӦ������������������Һ��Ӧ�����ܺ���ˮ��Ӧ����AΪNaHSO3�������ʾ��л�ԭ�ԣ����Ա���ˮ����Ϊ�����ƣ�ͬʱ�������ᣬA����ˮ��Ӧ�����ӷ���ʽ��HSO3-+H2O+Cl2=SO42-+2Cl-+3H+��
(7)��ѧ����Ϊ����NH5�������ʣ���Ԥ������ˮ���ҷ�Ӧ�ų����壬����ˮ��Һ�������ԣ�Ӧ�ǰ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ��NH5+H2O=H2��+NH3H2O��Ҳ����дΪNH5+H2O=H2��+NH3��+H2O �� NH5=H2��+NH3����

����Ŀ��ij����С���Ա��ӡ������Ҵ����ʵıȽ�Ϊ����������ʵ��̽���л�����������еĻ��������ʵĹ�ϵ���Լ�����֮������Ӱ�졣
��1�����ӡ������Ҵ��������ʱȽϣ���ˮ����Ϊ��
�Ҵ� | �� | ���� | |
ˮ���� | ��ˮ������Ȼ��� | ������ˮ | �����£���ˮ���ܽ����9.3g�����¶ȸ���65��ʱ������ˮ���� |
�Է������ӡ������Ҵ�ˮ���Բ����ԭ��______��
��2��ʵ�鷢�ֱ������������Ʒ�Ӧ�����ۼ�����ʵ�鷢�֣������±��ӵı�����ҺpH��5����ʱ��������ˮpH��5��6�����ǵ������ж�����̼��Ӱ�죬��С����Ϊ���һ��ʵ����ȷ�ϱ��ӵ����ԡ�
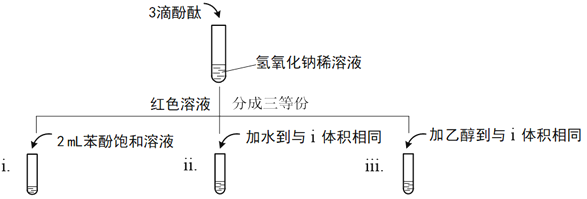
��д���������������Ʒ�Ӧ�Ļ�ѧ����ʽ______��
��֤�����������Ե�ʵ��������______��
�۱��������ԡ��Ҵ��������ԣ������е��ǻ�������������Ϊ______��
��3�����ʵ�鲢���ʵ������֤���������ǻ��Ա�����Ӱ��______����������������ͼ�Ⱦ��ɣ�
����Ŀ��![]() ʱ����Ũ��Ϊ
ʱ����Ũ��Ϊ![]() ������������Һ�ֱ�ζ�
������������Һ�ֱ�ζ�![]() Ũ�Ⱦ�Ϊ
Ũ�Ⱦ�Ϊ![]() �Ķ�����HX��
�Ķ�����HX��![]() ��������仯
��������仯![]() ��ʵ���������±��������ж���ȷ����
��ʵ���������±��������ж���ȷ����![]()
���ݱ�� | NaOH�������� | ��Һ��pH | |
|
| ||
�� | 0 | 3 | 1 |
�� | a | 7 | |
�� |
| x | y |
A.����ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��![]()
B.�ɱ������ݿɹ����![]()
C.HY��HX��ϣ�![]()
D.�����ڷ�Ӧ���HY��Һ�У�![]()
����Ŀ���ζ��ķ���������к͵ζ��������ζ�����ϵζ��ȣ������ζ����õ�ָʾ����������һ�ֳ���������֪һЩ���ε���ɫ�� ![]() ���£��ⶨˮ�����Ȼ���ĺ��������ñ���������Һ���еζ���
���£��ⶨˮ�����Ȼ���ĺ��������ñ���������Һ���еζ���
��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
Ksp | 1.8��10-10 | 5.0��10-13 | 8.3��1017 | 2.0��10-48 | 1.8��10-10 |
�ζ�ʱ������Ϊ�õζ�����ѡ�õ�ָʾ���������е�
A.KIB.![]() C.KBrD.
C.KBrD.![]()