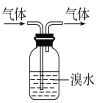
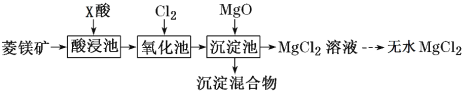
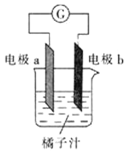
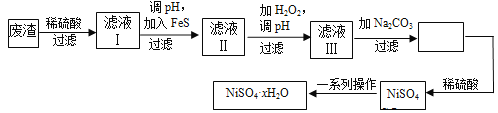
ЬтФПФкШн
ЁОЬтФПЁПNiSO4ЁЄxH2OЪЧвЛжжТЬЩЋвзШмгкЫЎЕФОЇЬхЃЌЙуЗКгУгкЖЦФјЁЂЕчГиЕШЃЌПЩгЩЕчЖЦЗЯдќЃЈГ§ФјЭтЃЌЛЙКЌгаЭЁЂаПЁЂЬњЕШдЊЫиЃЉЮЊдСЯЛёЕУЁЃВйзїВНжшШчЯТЃК
ЃЈ1ЃЉдкД§ЖЦМўЩЯЖЦФјЪБЃЌД§ЖЦМўгІзї_____МЋЃЌЕчЖЦЙ§ГЬжаЕчНтжЪШмвКХЈЖШ _____ЃЈЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЁЂЁАВЛБфЁБЃЉ
ЃЈ2ЃЉЯђТЫвКЂёжаМгШыFeSЪЧЮЊСЫГ§ШЅCu2ЃЋЁЂZn2ЃЋЕШдгжЪЃЌдђГ§ШЅCu2ЃЋЕФРызгЗНГЬЪНЮЊ_____________ЁЃЕБZn2ЃЋЧЁКУГСЕэЭъШЋЪБЃЌдкCuSЁЂZnSЙВДцЕФЛьКЯвКжаc(Zn2+)=10-5mol/L ,дђc(Cu2+)=_____mol/L ЃЈвбжЊKsp(CuS)=1.3ЁС10-36ЃЌKsp(ZnS)=1.6ЁС10-24ЃЉЁЃ
ЃЈ3ЃЉЖдТЫвКЂђжаЯШМгH2O2дйЕїpHЃЌЕїpHЕФФПЕФЪЧ__________________________ЁЃ
ЃЈ4ЃЉТЫвКЂѓШмжЪЕФжївЊГЩЗжЪЧNiSO4ЃЌМгШыNa2CO3Й§ТЫКѓдйМгЪЪСПЯЁСђЫсШмНтгжЩњГЩNiSO4ЃЌетСНВНВйзїЕФФПЕФЪЧ______________________________ЁЃ
ЃЈ5ЃЉЮЊВтЖЈNiSO4ЁЄxH2OОЇЬхxЕФжЕЃЌГЦШЁ26.3 gОЇЬхМгШШжСГфШЋЪЇШЅНсОЇЫЎЃЌЪЃгрЙЬЬх15.5 gЃЌдђxЕФжЕЕШгк___________ЁЃ
ЁОД№АИЁПвѕВЛБфFeS+Cu2ЃЋ=CuS+Fe2ЃЋ8.1ЁС10-18Г§ШЅ Fe3ЃЋдіДѓNiSO4ЕФХЈЖШЃЌРћгкеєЗЂНсОЇЃЈ(ЛђИЛМЏNiSO4ЃЉ6
ЁОНтЮіЁП
ЪдЬтЃЈ1ЃЉИљОнЕчЖЦдРэЗжЮіЃЛ
ЃЈ2ЃЉFeSГ§ШЅCu2+ЕФЗДгІЪЧГСЕэЕФзЊЛЏЃЛИљОнШмЖШЛ§ГЃЪ§МЦЫуЃЛ
ЃЈ3ЃЉИљОнЫЋбѕЫЎФмбѕЛЏбЧЬњРызгЗжЮіЃЛ
ЃЈ4ЃЉДгЗжРыЬсДПЕФНЧЖШЗжЮіЃЛ
ЃЈ5ЃЉИљОнжЪСПЪиКуЖЈТЩМЦЫуЁЃ
НтЮіЃКЃЈ1ЃЉдкД§ЖЦМўЩЯЖЦФјЪБЃЌД§ЖЦМўгІзївѕМЋМЋЃЌгЩгкбєМЋФјЪЇШЅЕчзгЃЌвѕМЋФјРызгЕУЕНЕчзгЃЌвђДЫЕчЖЦЙ§ГЬжаЕчНтжЪШмвКХЈЖШВЛБфЃЛ
ЃЈ2ЃЉFeSГ§ШЅCu2+ЕФЗДгІЪЧГСЕэЕФзЊЛЏЃЌЗДгІЕФРызгЗНГЬЪНЮЊЃКFeS+Cu2+=CuS+Fe2+ЃЛЕБZn2ЃЋЧЁКУГСЕэЭъШЋЪБЃЌдкCuSЁЂZnSЙВДцЕФЛьКЯвКжаДцдк![]() ЃЌШчЙћc(Zn2+)=10-5mol/LЃЌдђc(Cu2+)=8.1ЁС10-18 mol/LЁЃ
ЃЌШчЙћc(Zn2+)=10-5mol/LЃЌдђc(Cu2+)=8.1ЁС10-18 mol/LЁЃ
ЃЈ3ЃЉЖдТЫвКЂђМгH2O2ЕФФПЕФЪЧНЋFe2+бѕЛЏFe3+ЃЌШЛКѓЕїpHЪЙFe3+ЭъШЋГСЕэЃЛ
ЃЈ4ЃЉNiSO4гыNa2CO3ЗДгІЩњГЩNiCO3ГСЕэЃЌЖјКѓЙ§ТЫЃЌдйМгЪЪСПЯЁСђЫсШмНтгжЩњГЩNiSO4ЃЌетбљПЩЬсИпNiSO4ЕФХЈЖШЃЌгаРћгкеєЗЂНсОЇЃЛ
ЃЈ5ЃЉВтЖЈNiSO4xH2OОЇЬхжаxЕФжЕЃЌГЦШЁ26.3gОЇЬхМгШШжСЭъШЋЪЇШЅНсОЇЫЎЃЌЪЃгрЙЬЬх15.5gЃЌЪЇШЅЫЎЕФжЪСП=26.3g-15.5g=10.8gЃЌNiSO4ЕФжЪСПЮЊ15.5gЃЌМЦЫуЮяжЪЕФСПЕУЕН1ЃКx=15.5g/155g/molЃК10.8g/18g/molЃЌНтЕУx=6ЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПШШЛЏбЇКЭИїРрЦНКтЕШЖМЪЧЛЏбЇбаОПЕФЖдЯѓ
(1)вбжЊЃКЂй2O2(g)ЃЋN2(g)ЃНN2O4(l) ІЄH1ЃЛ
ЂкN2(g)ЃЋ2H2(g)ЃНN2H4(g) ІЄH2ЃЛ
ЂлO2(g)ЃЋ2H2(g)ЃН2H2O(g) ІЄH3ЃЛ
Ђм2N2H4(g)ЃЋN2O4(l)ЃН3N2(g)ЃЋ4H2O(g) ІЄH4
ЩЯЪіЗДгІШШаЇгІжЎМфЕФЙиЯЕЪНЮЊІЄH4 ЃН___________(гУКЌІЄH1ЁЂІЄH2ЁЂІЄH3ЕФДњЪ§ЪНБэЪО)
(2)НЋВЛЭЌСПЕФH2O(g)КЭCO(g)ЗжБ№ЭЈШыЬхЛ§ЮЊ2LЕФКуШнУмБеШнЦїжаЃЌНјааЗДгІЃКH2O(g)+CO(g)H2(g)+CO2(g)ЃЌЕУЕНШчЯТБэШ§зщЪ§ОнЃК
ЪЕбщзщ | ЮТЖШ/Ёц | Ц№ЪМСП/mol | ЦНКтСП/mol | ДяЕНЦНКтЫљашЪБМф/min | ||
H2O | CO | H2 | CO | |||
1 | 800 | 2 | 4 | 4/3 | 8/3 | 6 |
2 | 900 | 1 | 2 | 0.4 | 0.6 | 3 |
3 | 900 | a | b | c | d | t |
Ђй ИУЗДгІЮЊ________(ЬюЁАЮќШШЁБЛђЁАЗХШШЁБ)ЗДгІЃЛЪЕбщ2ЕФЦНКтГЃЪ§K= _________ЁЃ
Ђк ШєЪЕбщ3ДяЕНЦНКтЪБгыЪЕбщ2ДяЕНЦНКтзДЬЌЪБИїЮяжЪЕФЬхЛ§ЗжЪ§ЗжБ№ЯрЕШЃЌЧвt<3ЃЌдђaЁЂbгІТњзуЕФЙиЯЕЪЧ_______(гУКЌaЁЂbЕФДњЪ§ЪНБэЪО)ЁЃ
ЂлШєБЃГжЮТЖШКЭШнЛ§ВЛБфЃЌЯђЪЕбщ1дйдіМг4mol H2O(g)ЃЌЪЙЗДгІДяЕНаТЦНКтЃЌЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ_______ЁЃ
A.аТОЩЦНКтЪБШнЦїЦјЬхбЙЧПжЎБШЮЊ5:3
B.аТЦНКтЪБH2OЕФзЊЛЏТЪдіДѓ
C.аТЦНКтЪБCOЕФХЈЖШЪЧ0.8 molЁЄL-1
D.аТОЩЦНКтЪБШнЦїЦјЬхУмЖШжЎБШЮЊ5:3
(3)ЪвЮТЯТЃЌгУ0.1 molЁЄL-1ЕФKOHШмвКЕЮЖЈ10.00 mL 0.10 molЁЄL-1H2C2O4 (ЖўдЊШѕЫс)ШмвКЃЌЫљЕУЕЮЖЈЧњЯпШчЭМ(ЛьКЯШмвКЕФЬхЛ§ПЩПДГЩЛьКЯЧАШмвКЕФЬхЛ§жЎКЭ)ЁЃЧыЛиД№ЯТСаЮЪЬтЃК
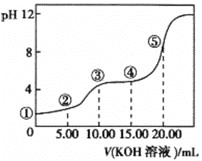
ЂйЕуЂкЫљЪОЕФШмвКжаЕФЕчКЩЪиКуЪНЮЊ_____________ЁЃ
ЂкЕуЂлЫљЪОШмвКжаИїРызгХЈЖШгЩДѓЕНаЁЕФЫГађЮЊ___________ЁЃ
ЂлЕуЂмЫљЪОШмвКжаc(K+) + c(H2C2O4 ) + c(![]() ) + c(
) + c(![]() ) =_______ molЁЄL-1ЁЃ
) =_______ molЁЄL-1ЁЃ