ĚâÄżÄÚČÝ
ˇľĚâÄżˇżĚúĽ°Ć仯şĎÎďÔÚÓĐ»ú»ŻŃ§ÖĐÓ¦ÓĂąă·şŁ¬ŔýČçÓĐ»úşĎłÉÖĐŁ¬łŁÓĂĚúşÍŃÎËὫĎő»ů(ŁNO2)»ąÔÎŞ°±»ů(ŁNH2)Ł»ÔÚ±˝µÄäĺ´ú·´Ó¦ÖĐÓĂä廯Ěú×÷´ß»ŻĽÁˇŁ
(1)Fe»ů̬Ô×ÓşËÍâµç×ÓĹŲĽĘ˝ÎŞ________________________ˇŁ
(2)Hˇ˘Nˇ˘OµÄµç¸şĐÔ´ÓСµ˝´óµÄËłĐňĘÇ_________________ˇŁ
(3)ÓëNO![]() »ĄÎŞµČµç×ÓĚĺµÄŇ»ÖÖ·Ö×ÓÎŞ__________(Ěѧʽ)ˇŁ°±»ů(ŁNH2)ÖеŞÔ×ÓµÄÔÓ»ŻŔŕĐÍÎŞ_____________ˇŁ
»ĄÎŞµČµç×ÓĚĺµÄŇ»ÖÖ·Ö×ÓÎŞ__________(Ěѧʽ)ˇŁ°±»ů(ŁNH2)ÖеŞÔ×ÓµÄÔÓ»ŻŔŕĐÍÎŞ_____________ˇŁ
(4)1mol±˝°··Ö×ÓÖĐş¬ÓЦҼüµÄĘýĿΪ_____________________ˇŁ
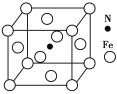
(5)FeÓëNĐγɵÄÄł»ŻşĎÎᄃ°űČçÍĽËůĘľŁ¬Ôň¸Ăľ§ĚĺµÄ»ŻŃ§Ę˝ÎŞ______________ˇŁ
ˇľ´đ°¸ˇż[Ar]3d64s2 HŁĽNŁĽO N2O (»ňCO2ˇ˘CS2µČ) sp3 14 mol Fe4N
ˇľ˝âÎöˇż
(1)¸ůľÝĚúµÄÔ×ÓĐňĘýÎŞ26Ł¬˝áşĎÄÜÁż×îµÍÔŔíĘéĐ´»ů̬Ô×ӵĵç×ÓĹŲĽĘ˝Ł»
(2)¸ůľÝµç¸şĐԵı仯ąćÂɱȽϵ縺ĐÔ´óСŁ»
(3)NO![]() µÄÔ×Ó×ÜĘýÎŞ3ˇ˘ĽŰµç×Ó×ÜĘýÎŞ16Ł¬˝áşĎµČµç×ÓĚĺµÄ¸ĹÄî·ÖÎö˝â´đŁ»°±»ů(-NH2)ÖеŞÔ×ÓĐÎłÉ3¸ö¦ŇĽüŁ¬Ň»¸öąÂ¶Ôµç×ÓŁ¬ľÝ´ËĹжϣ»
µÄÔ×Ó×ÜĘýÎŞ3ˇ˘ĽŰµç×Ó×ÜĘýÎŞ16Ł¬˝áşĎµČµç×ÓĚĺµÄ¸ĹÄî·ÖÎö˝â´đŁ»°±»ů(-NH2)ÖеŞÔ×ÓĐÎłÉ3¸ö¦ŇĽüŁ¬Ň»¸öąÂ¶Ôµç×ÓŁ¬ľÝ´ËĹжϣ»
(4)¸ůľÝ±˝°·(![]() )·Ö×ӵĽṹ·ÖÎö˝â´đŁ»
)·Ö×ӵĽṹ·ÖÎö˝â´đŁ»
(5)¸ůľÝľůĚŻ·¨·ÖÎöĹжϺ¬ÓĐFeşÍNµÄÔ×Ó¸öĘýŁ¬ÔŮĹжϸþ§ĚĺµÄ»ŻŃ§Ę˝ˇŁ
(1)ĚúµÄÔ×ÓĐňĘýÎŞ26Ł¬¸ůľÝÄÜÁż×îµÍÔŔíŁ¬Ćä»ů̬Ô×ӵĵç×ÓĹŲĽĘ˝ÎŞ1s22s22p63s23p63d64s2»ň[Ar]3d64s2Ł¬ąĘ´đ°¸ÎŞŁş1s22s22p63s23p63d64s2»ň[Ar]3d64s2Ł»
(2)ÔÚÔŞËŘÖÜĆÚ±íÖĐŁ¬Í¬Ň»ÖÜĆÚ´Ó×óµ˝ÓŇŁ¬ÔŞËصĵ縺ĐÔÖđ˝ĄÔö´óŁ¬Í¬Ň»Ö÷×ĺ´ÓÉϵ˝ĎÂŁ¬ÔŞËصĵ縺ĐÔÖ𽥼őČőŁ¬ÔŞËصķǽđĘôĐÔԽǿˇ˘µç¸şĐÔÔ˝´óŁ¬µç¸şĐÔ´ÓСµ˝´óµÄËłĐňÎŞHŁĽNŁĽOŁ¬ąĘ´đ°¸ÎŞŁşHŁĽNŁĽOŁ»
(3)NO![]() µÄÔ×Ó×ÜĘýÎŞ3ˇ˘ĽŰµç×Ó×ÜĘýÎŞ16Ł¬ĆäµČµç×ÓĚĺÎŞN2O(»ňCO2ˇ˘CS2µČ)Ł»°±»ů(-NH2)ÖеŞÔ×ÓĐÎłÉ3¸ö¦ŇĽüŁ¬Ň»¸öąÂ¶Ôµç×ÓŁ¬ĽŰ˛ăµç×Ó¶ÔĘýÎŞ4Ł¬µŞÔ×ÓÎŞsp3ÔÓ»ŻŁ¬ąĘ´đ°¸ÎŞŁşN2O(»ňCO2ˇ˘CS2µČ)Ł»sp3Ł»
µÄÔ×Ó×ÜĘýÎŞ3ˇ˘ĽŰµç×Ó×ÜĘýÎŞ16Ł¬ĆäµČµç×ÓĚĺÎŞN2O(»ňCO2ˇ˘CS2µČ)Ł»°±»ů(-NH2)ÖеŞÔ×ÓĐÎłÉ3¸ö¦ŇĽüŁ¬Ň»¸öąÂ¶Ôµç×ÓŁ¬ĽŰ˛ăµç×Ó¶ÔĘýÎŞ4Ł¬µŞÔ×ÓÎŞsp3ÔÓ»ŻŁ¬ąĘ´đ°¸ÎŞŁşN2O(»ňCO2ˇ˘CS2µČ)Ł»sp3Ł»
(4)±˝°·(![]() )·Ö×ÓÖĐĚĽĚĽÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛6¸öŁ»ĚĽÇâÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛5¸öŁ¬ĚĽµŞÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛1¸öŁ¬µŞÇâÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛2¸öŁ¬ËůŇÔ1mol±˝°··Ö×ÓÖĐş¬ÓЦҼüµÄĘýĿΪ14mol(»ň14ˇÁ6.02ˇÁ1023)Ł¬ąĘ´đ°¸ÎŞŁş14mol(»ň14ˇÁ6.02ˇÁ1023)Ł»
)·Ö×ÓÖĐĚĽĚĽÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛6¸öŁ»ĚĽÇâÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛5¸öŁ¬ĚĽµŞÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛1¸öŁ¬µŞÇâÖ®Ľäş¬ÓĐŇ»¸ö¦ŇĽüŁ¬ą˛2¸öŁ¬ËůŇÔ1mol±˝°··Ö×ÓÖĐş¬ÓЦҼüµÄĘýĿΪ14mol(»ň14ˇÁ6.02ˇÁ1023)Ł¬ąĘ´đ°¸ÎŞŁş14mol(»ň14ˇÁ6.02ˇÁ1023)Ł»
(5)Ôڸþ§°űÖĐŁ¬ş¬ÓĐFeŁş8ˇÁ![]() +6ˇÁ
+6ˇÁ![]() =4Ł¬NÎŞ1¸öŁ¬Ôň¸Ăľ§ĚĺµÄ»ŻŃ§Ę˝ÎŞFe4NŁ¬ąĘ´đ°¸ÎŞŁşFe4NˇŁ
=4Ł¬NÎŞ1¸öŁ¬Ôň¸Ăľ§ĚĺµÄ»ŻŃ§Ę˝ÎŞFe4NŁ¬ąĘ´đ°¸ÎŞŁşFe4NˇŁ

ˇľĚâÄżˇżµŞĘǵŘÇňÉĎş¬Áż·á¸»µÄŇ»ÖÖÔŞËŘŁ¬µŞÔŞËصĵĄÖĘĽ°Ć仯şĎÎďÔÚą¤Ĺ©ŇµÉú˛úˇ˘Éú»îÖĐÓĐ×ĹÖŘŇŞ×÷ÓáŁ
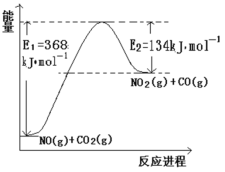
˘Ĺ¸ůľÝĎÂĂćÄÜÁż±ä»ŻĘľŇâÍĽŁ¬ÇëĐ´łö NO şÍ CO2·´Ó¦µÄČČ»ŻŃ§·˝łĚĘ˝_________ˇŁ
˘ĆÔڹ̶¨Ěĺ»ýµÄĂܱŐČÝĆ÷ÖĐŁ¬˝řĐĐČçĎ»ŻŃ§·´Ó¦Łş2NH3(g)![]() N2(g)+3H2(g) ˇ÷HŁľ0Ł¬ĆäĆ˝şâłŁĘý K ÓëÎÂ¶Č T µÄąŘϵČçĎÂ±íŁş
N2(g)+3H2(g) ˇ÷HŁľ0Ł¬ĆäĆ˝şâłŁĘý K ÓëÎÂ¶Č T µÄąŘϵČçĎÂ±íŁş
T/K | 298 | 398 | 498 |
Ć˝şâłŁĘýK | 4.1ˇÁ106 | K1 | K2 |
˘ŮĘÔĹĐ¶Ď K1_________K2Ł¨ĚîĐ´ˇ°Łľˇ±Ł¬ˇ°ŁĽˇ±»ňˇ°Ł˝ˇ±Ł©Ł»
˘Ú¸Ă·´Ó¦µÄěرäS_________0Ł¨Ěî >ˇ˘< »ň = Ł©
˘ŰĎÂÁи÷ĎîÄÜ˵Ă÷¸Ă·´Ó¦ŇѴﵽƽşâ״̬µÄĘÇ_________Ł¨Ěî×ÖĸŁ©Ł»
aŁ®ČÝĆ÷ÄÚN2ˇ˘H2ˇ˘NH3µÄÎďÖʵÄÁżÖ®±ČÎŞ1ˇĂ3ˇĂ2 bŁ®2¦Ô(NH3)Őý Ł˝ 3¦Ô(H2)Äć
cŁ®ČÝĆ÷ÄÚ»ěşĎĆřĚĺĆ˝ľůĎŕ¶Ô·Ö×ÓÖĘÁż±ŁłÖ˛»±ä dŁ®»ěşĎĆřĚĺµÄĂܶȱŁłÖ˛»±ä
˘ÜŇ»¶¨Î¶ČĎÂŁ¬ÔÚ 1L ĂܱŐČÝĆ÷ÖĐłäČë 1molN2şÍ 3molH2 ˛˘·˘Éú·´Ó¦ˇŁČôČÝĆ÷ČÝ»ýşă¶¨Ł¬10min ´ďµ˝Ć˝şâʱŁ¬ĆřĚĺµÄ×ÜÎďÖʵÄÁżÎŞÔŔ´µÄ9/10Ł¬ÔňN2µÄת»ŻÂĘ![]() (N2)=_________________
(N2)=_________________
˘Ç¶Ô·´Ó¦ N2O4Ł¨gŁ©![]() 2NO2Ł¨gŁ© ˇ÷H > 0 Ł¬ÔÚζȷֱđÎŞT1ˇ˘T2 ʱŁ¬Ć˝şâĚĺϵÖĐ NO2µÄĚĺ»ý·ÖĘýËćѹǿ±ä»ŻÇúĎßČçÓŇÍĽËůĘľŁ¬ĎÂÁĐ˵·¨ŐýČ·µÄĘÇ__________ˇŁ
2NO2Ł¨gŁ© ˇ÷H > 0 Ł¬ÔÚζȷֱđÎŞT1ˇ˘T2 ʱŁ¬Ć˝şâĚĺϵÖĐ NO2µÄĚĺ»ý·ÖĘýËćѹǿ±ä»ŻÇúĎßČçÓŇÍĽËůĘľŁ¬ĎÂÁĐ˵·¨ŐýČ·µÄĘÇ__________ˇŁ
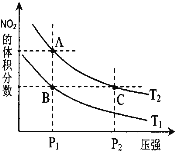
aŁ®Aˇ˘C Á˝µăµÄ·´Ó¦ËŮÂĘŁşAŁľC
bŁ®Aˇ˘C Á˝µăµÄ»ŻŃ§Ć˝şâłŁĘýŁşAŁľC
cŁ®Aˇ˘C Á˝µă N2O4µÄת»ŻÂĘŁşAŁľC
dŁ®ÓÉ״̬ B µ˝×´Ě¬ AŁ¬żÉŇÔÓĂĽÓČȵķ˝·¨
ˇľĚâÄżˇżłŁÎÂĎÂŁ¬˝«Ň»¶¨Ĺ¨¶ČµÄHAşÍHB·Ö±đÓë0.10mol/LµÄNaOHČÜŇşµČĚĺ»ý»ěşĎŁ¬ĘµŃéĽÇÂĽČçĎÂ±íŁş
ʵŃé±ŕşĹ | ĽÓČëµÄËá | ĽÓČëËáµÄŨ¶Č/(mol/L) | »ěşĎşóČÜŇşµÄpH |
˘Ů | HA | 0.10 | 8.7 |
˘Ú | HB | 0.12 | 2 |
ĎÂÁĐ˵·¨ÖĐŐýČ·µÄĘÇ
A. HAĘÇÇżËᣬHBĘÇČőËá
B. Éý¸ßζȣ¬ČÜŇş˘ÚÖĐc(B-)/c(Na+Ł©Ôö´ó
C. ČÜŇş˘ŮÖĐŔë×ÓŨ¶ČµÄąŘϵŁşc(A-)>c(Na+)>c(OH->c(H+Ł©
D. ČÜŇş˘ÚÖĐŔë×ÓŨ¶ČµÄąŘϵŁşc(Na+)Ł«c(H+Ł«c(B-)=0.12 mol/L
ˇľĚâÄżˇżŇŇËáŇěÎěőĄĘÇ×éłÉĂŰ·äĐĹϢËصijɷÖ֮һŁ¬ľßÓĐĎ㽶µÄĎăζˇŁĘµŃéĘŇÖƱ¸ŇŇËáŇěÎěőĄµÄ·´Ó¦ˇ˘×°ÖĂĘľŇâÍĽşÍÓĐąŘĘýľÝČçĎÂŁş
![]() +
+![]()
![]()
![]() +H2O
+H2O
Ďŕ¶Ô·Ö×ÓÖĘÁż | ĂܶČ/(gˇ¤cm-3) | ·Đµă/ˇć | Ë®ÖĐČÜ˝âĐÔ | |
ŇěÎě´Ľ | 88 | 0.8123 | 131 | ΢ČÜ |
ŇŇËá | 60 | 1.0492 | 118 | ČÜ |
ŇŇËáŇěÎěőĄ | 130 | 0.8670 | 142 | ÄŃČÜ |

ʵŃ鲽Ö裺
ÔÚAÖĐĽÓČë4.4 gŇěÎě´Ľˇ˘6.0 gŇŇËᡢĘýµÎŨÁňËáşÍ2ˇ«3ƬËé´ÉƬˇŁżŞĘĽ»şÂýĽÓČČAŁ¬»ŘÁ÷50 minˇŁ·´Ó¦ŇşŔäÖÁĘŇÎÂşóµąČë·ÖҺ©¶·ÖĐŁ¬·Ö±đÓĂÉŮÁżË®ˇ˘±ĄşÍĚĽËáÇâÄĆČÜŇşşÍˮϴµÓŁ»·ÖłöµÄ˛úÎďĽÓČëÉŮÁżÎŢË®MgSO4ąĚĚ壬ľ˛ÖĂƬżĚŁ¬ąýÂËłýČĄMgSO4ąĚĚ壬˝řĐĐŐôÁó´ż»ŻŁ¬ĘŐĽŻ140ˇ«143ˇćÁó·ÖŁ¬µĂŇŇËáŇěÎěőĄ3.9 gˇŁ
»Ř´đĎÂÁĐÎĘĚ⣺
(1)ŇÇĆ÷BµÄĂűłĆĘÇ______ Ł¬Ćä×÷ÓĂĘÇ_______ˇŁ
(2)ÔÚĎ´µÓ˛Ů×÷ÖĐŁ¬µÚŇ»´ÎˮϴµÄÖ÷ŇŞÄżµÄĘÇ________Ł¬µÚ¶ţ´ÎˮϴµÄÖ÷ŇŞÄżµÄĘÇ__________________ˇŁ
(3)ÔÚĎ´µÓˇ˘·ÖŇş˛Ů×÷ÖĐŁ¬Ó¦łä·ÖŐńµ´Ł¬Č»şóľ˛ÖĂŁ¬´ý·Ö˛ăşó_____(Ěî±ęşĹ)ˇŁ
a.Ö±˝Ó˝«ŇŇËáŇěÎěőĄ´Ó·ÖҺ©¶·µÄÉĎżÚµąłö
b.Ö±˝Ó˝«ŇŇËáŇěÎěőĄ´Ó·ÖҺ©¶·µÄĎÂżÚ·Ĺłö
c.ĎČ˝«Ë®˛ă´Ó·ÖҺ©¶·µÄĎÂżÚ·ĹłöŁ¬ÔŮ˝«ŇŇËáŇěÎěőĄ´ÓĎÂżÚ·Ĺłö
d.ĎČ˝«Ë®˛ă´Ó·ÖҺ©¶·µÄĎÂżÚ·ĹłöŁ¬ÔŮ˝«ŇŇËáŇěÎěőĄ´ÓÉĎżÚµąłö
(4)±ľĘµŃéÖĐĽÓČëąýÁżŇŇËáµÄÄżµÄĘÇ________ˇŁ
(5)ʵŃéÖĐĽÓČëÉŮÁżÎŢË®MgSO4µÄÄżµÄĘÇ___________ˇŁ
(6)±ľĘµŃéµÄ˛úÂĘĘÇ________ˇŁ
(7)ÔÚ˝řĐĐŐôÁó˛Ů×÷ʱŁ¬Čô´Ó130ˇćżŞĘĽĘŐĽŻÁó·ÖŁ¬»áʹʵŃéµÄ˛úÂĘĆ«_____(Ě¸ßˇ±»ňˇ°µÍˇ±)Ł¬ÔŇňĘÇ__________ˇŁ