��Ŀ����
��������Ҫ�ɷ�ΪFeO��Cr2O3������������Al2O3��һ���������Cr2O3��������ԼΪ40�����ɸ������Ʊ��ظ���صķ������£�
��֪��
��4FeO��Cr2O3��8Na2CO3��7O2 8Na2CrO4��2 Fe2O3��8CO2����
8Na2CrO4��2 Fe2O3��8CO2����
��Na2CO3��Al2O3 2NaAlO2��CO2����
2NaAlO2��CO2����
�� Cr2O72����H2O  2CrO42����2H+
2CrO42����2H+
��������ش��������⣺
��1������IΪ ������X����Ҫ���� ����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ�� ����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH<5����Ŀ���� ��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ� �����
��4������Y����Ҫ����������������д��������Һ��pH=7~8ʱ�����������������ӷ���ʽ ��
��1�����ˣ�2�֣��� Fe2O3��2�֣��� pH�ƻ���pH��ֽ�Ͳ�������2�֣���
��2������H+Ũ�ȿ�ʹ��ѧƽ��Cr2O72����H2O  2CrO42����2H+�����ƶ���ʹCrO42��ת��ΪCr2O72���������ش�����֣���2�֣���
2CrO42����2H+�����ƶ���ʹCrO42��ת��ΪCr2O72���������ش�����֣���2�֣���
��3����ȴ�ᾧ��2�֣��� ϴ�ӣ�2�֣���
��4��CH3COOH��AlO2����H2O = Al(OH)3����CH3COO�� ��2�֣�
��������������������պ�IJ�����Na2CrO4��Fe2O3��NaAlO2����ˮ��ȡ��������ˮ����Fe2O3��Ҳ����X�����˷��룬�Ӵ����PH��7~8��ʹAlO2-ת��ΪAl(OH)3���������˷��룬Ҳ����Y��������PH<5��ʹCrO42��ת��ΪCr2O72������KCl�����ᾧ�ȵ��ظ����
���㣺���黯ѧ�������̡����������ʡ�����ʵ�����

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д������й�˵���У�����ȷ����
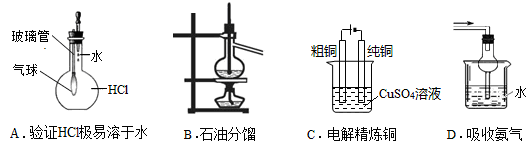
| A����ȥFe(OH)3������������NaCl�����������ķ��� |
| B��ʵ���������������ơ��س������������� |
| C���к͵ζ�ʱҪ���ζ���ϴ�����ô�ʢ��Һ��ϴ2��3�� |
| D�������ᡢKSCN��Һ��Fe(OH)3��Һ��Ϻ���Һ�Ժ�ɫ |
���и���ʵ��������������ó��Ľ�����ȷ����
| ѡ�� | ʵ����� | ʵ������ | �� �� |
| A | ����ҺX���ȵμ�ϡ���ᣬ�ٵμ�Ba(NO3)2��Һ | ���ְ�ɫ���� | ��ҺX��һ������SO42�� |
| B | �����pH��3��HA��HB������ֱ���������п��Ӧ����ˮ���ռ����� | HA�ų����������ҷ�Ӧ���ʿ� | HB���Ա�HAǿ |
| C | ��������NaOH��Һ���Ⱥμ�AgNO3��Һ | δ���ֵ���ɫ���� | ������δ����ˮ�� |
| D | ��1mL1����NaOH��Һ�м���2mL2%��CuSO4��Һ�����ټ���0.5mL�л���Y������ | δ����ש��ɫ���� | Y�в�����ȩ�� |
����ʵ����ȷ����
| A������Ͳ��ȡ15.50mL 0.100 mol��L��1���� |
| B������ˮ�ƾ���ȡ��ˮ�еĵ� |
| C���ù���Ũ��ˮϴ���Թ��ڵ����� |
| D����H2��ԭCuOʵ��ʱ��Ҫ��ͨ�������鴿���ټ��� |