��Ŀ����
��15�֣�ij�о�С���û�ͭ����Ҫ�ɷ���CuFeS2������SΪ-2�ۣ�Ϊ��Ҫԭ����ͭ�����ܷ�ӦΪ��2CuFeS2+2SiO2+5O2��2Cu+2FeSiO3+4SO2����ʵ�ϸ÷�Ӧ�ǰ��������̷ֲ����еģ�


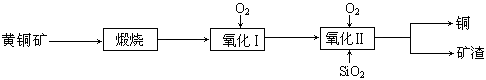
��1��������ķ�Ӧ��Ҫ���������ɵ�����������һ������Ϊ��������������������跴Ӧ���ɿ�������������Ҫ�ɷ��� ���ѧʽ����
��2���ݱ�������һ��ϸ�������������¿��Խ���ͭ�������������Σ���Ӧ����������Һ�з����ġ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3���ҹ�ѧ���о����֣��Ծ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ����������ȴ���ܽ⡢�������ᾧ���õ�CuSO4��5H2O�������ɱ��ܹ��������ࡣ�й�ʵ�������±���
��CuFeS2��O2��Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ�����������з���¯�¶�Ϊ600��620 �档������¶ȵķ����� ��
�۵��¶ȸ���600��620 ��ʱ����������ˮ����ͭ�½���ԭ���� ��
����������ȴ��ij�����ʵ�����������Ҫ�� ����֪����Һ�У�Cu2+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.7��6.7��Fe3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.1��3.2������Ƶõ�����ͭ��Һ�к���������Fe3+����д����ȥ��Һ��Fe3+��ʵ��������裺 ��



��1��������ķ�Ӧ��Ҫ���������ɵ�����������һ������Ϊ��������������������跴Ӧ���ɿ�������������Ҫ�ɷ��� ���ѧʽ����
��2���ݱ�������һ��ϸ�������������¿��Խ���ͭ�������������Σ���Ӧ����������Һ�з����ġ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3���ҹ�ѧ���о����֣��Ծ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ����������ȴ���ܽ⡢�������ᾧ���õ�CuSO4��5H2O�������ɱ��ܹ��������ࡣ�й�ʵ�������±���
| ����¯�¶�/�� | 560 | 580 | 600 | 620 | 640 | 660 |
| ˮ����Cu/% | 90.12 | 91.24 | 93.50 | 92.38 | 89.96 | 84.23 |
| ������Cu/% | 92.00 | 93.60 | 97.08 | 97.82 | 98.16 | 98.19 |
��ʵ�����������з���¯�¶�Ϊ600��620 �档������¶ȵķ����� ��
�۵��¶ȸ���600��620 ��ʱ����������ˮ����ͭ�½���ԭ���� ��
����������ȴ��ij�����ʵ�����������Ҫ�� ����֪����Һ�У�Cu2+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.7��6.7��Fe3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.1��3.2������Ƶõ�����ͭ��Һ�к���������Fe3+����д����ȥ��Һ��Fe3+��ʵ��������裺 ��
��15��
��1��FeSiO3��2�֣�
��2��4CuFeS2+2H2SO4+17O2 4CuSO4+2Fe2(SO4)3+2H2O��2�֣�
4CuSO4+2Fe2(SO4)3+2H2O��2�֣�
��3����4CuFeS2+15O2��4CuSO4+2Fe2O3+4SO2��2�֣�
�ڿ��Ƽ���CuFeS2���ٶȣ���CuFeS2��O2��Ӧ���ȣ���2�֣�
��CuSO4���ȷֽ⣨2�֣�
�ܹ��ˣ�2�֣� ����CuO[��Cu(OH)2��CuCO3]��ĩ����ֽ��裬������Һ��pHԼΪ3.2��������У����ˣ��ã�������ϡ�����ữ��3�֣�
��1��FeSiO3��2�֣�
��2��4CuFeS2+2H2SO4+17O2
 4CuSO4+2Fe2(SO4)3+2H2O��2�֣�
4CuSO4+2Fe2(SO4)3+2H2O��2�֣���3����4CuFeS2+15O2��4CuSO4+2Fe2O3+4SO2��2�֣�
�ڿ��Ƽ���CuFeS2���ٶȣ���CuFeS2��O2��Ӧ���ȣ���2�֣�
��CuSO4���ȷֽ⣨2�֣�
�ܹ��ˣ�2�֣� ����CuO[��Cu(OH)2��CuCO3]��ĩ����ֽ��裬������Һ��pHԼΪ3.2��������У����ˣ��ã�������ϡ�����ữ��3�֣�
�����������1������2CuFeS2+2SiO2+5O2��2Cu+2FeSiO3+4SO2���ɵÿ���ΪFeSiO3��
��2���������֪��CuFeS2��������Һ����������һ��ϸ�������������ɵ�������������ͭ������������ѧ����ʽΪ4CuFeS2+2H2SO4+17O2
 4CuSO4+2Fe2(SO4)3+2H2O��
4CuSO4+2Fe2(SO4)3+2H2O����3���پ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ�����յ�CuSO4��5H2O��˵����Ԫ�صĴ�����ʽ������������ˮ�ܽ�ɹ��˳�ȥ������CuFeS2��O2��Ӧ�Ļ�ѧ����ʽΪ4CuFeS2+15O2��4CuSO4+2Fe2O3+4SO2
����ΪCuFeS2��O2��Ӧ���ȣ����������������п��Ƽ���CuFeS2���ٶȣ������¶ȣ�
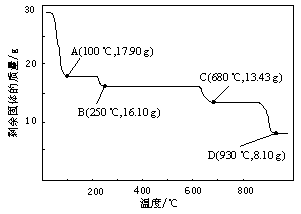
��ˮ����ͭ������ΪCuSO4?5H2O��������ͭ������ΪCuO���¶Ƚϸ�ʱ��CuSO4?5H2O�ɷֽ�����CuO������600������ʱˮ����ͭ�����ﺬ�����٣�
���������г�����ͭ�����������������������ˮ��������ȴ��ij�����ʵ�����������Ҫ�ǹ��ˣ���ȥ�����Ӷ�����ȥͭ���ӣ�������Һ��pHֵ��3.2��4.7֮�䣬ʹ��������ȫ��������ͭ���Ӳ����������Ծ�������Ǽ���CuO[��Cu(OH)2��CuCO3]��ĩ����ֽ��裬������Һ��pHԼΪ3.2��������У����ˣ��ã�������ϡ�����ữ��

��ϰ��ϵ�д�
�����Ŀ
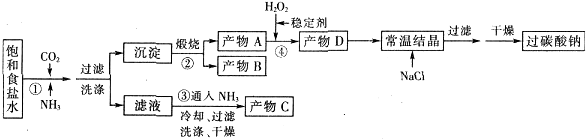
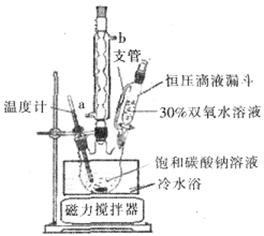 ��
��
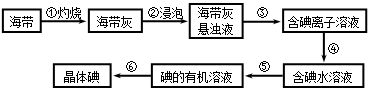
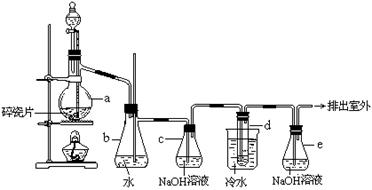
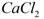 ��ij��������������¹������̣�
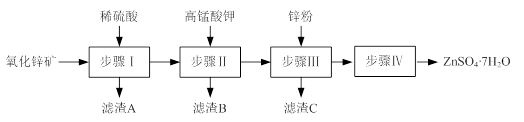
��ij��������������¹������̣�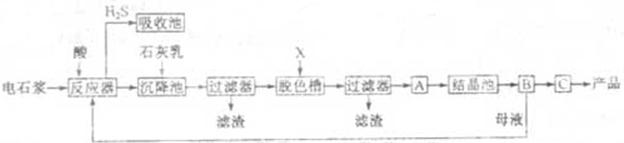
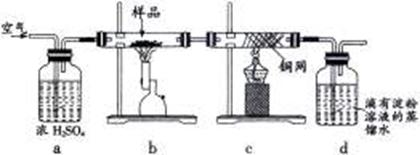
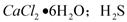 ��һ���������壬�Ҿ��л�ԭ�ԡ�
��һ���������壬�Ҿ��л�ԭ�ԡ�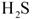 ͨ�����ճأ��������������ʺ���Ϊ���ռ����� �� ��
ͨ�����ճأ��������������ʺ���Ϊ���ռ����� �� ��