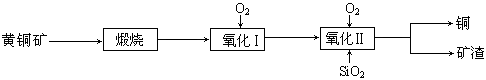
��Ŀ����
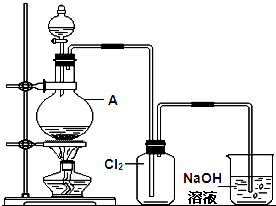
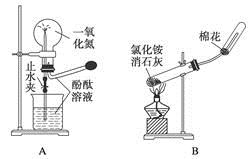
������ʾ���������ڴ����а���ȼ�ա�ijУ��ѧС��ѧ���������װ�ã�ͼ�����еȼг�װ������ȥ�����а����������ڲ�ͬ�����·�Ӧ��ʵ�飮
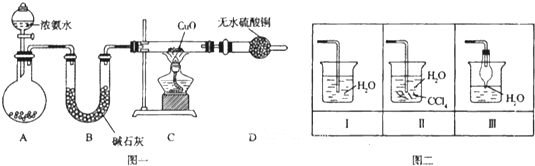
��1��ͼ����ʵ������ȡ����������İ�����װ��ͼ��д�����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��______���ܷ�����ˮCaCl2�����ʯ�ң�______����ܡ����ܡ�����
��2���������İ��������������ͨ��װ�����У��þƾ���Ƽ��Ⱥ��Թ�������Ϊ______ɫ�����������Ļ�ѧ����ʽ��______��
��3����������������ײ����İ����ֱ��a��b���ܽ�����ͨ�뵽װ�ñ��У�����b���϶˵�ȼ����������������Ϊ�����ɷ�֮һ����������ͨ����Ⱥ�˳����______���ڰ���ȼ�յĻ�ѧ����ʽ��______��

��1��ͼ����ʵ������ȡ����������İ�����װ��ͼ��д�����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��______���ܷ�����ˮCaCl2�����ʯ�ң�______����ܡ����ܡ�����
��2���������İ��������������ͨ��װ�����У��þƾ���Ƽ��Ⱥ��Թ�������Ϊ______ɫ�����������Ļ�ѧ����ʽ��______��
��3����������������ײ����İ����ֱ��a��b���ܽ�����ͨ�뵽װ�ñ��У�����b���϶˵�ȼ����������������Ϊ�����ɷ�֮һ����������ͨ����Ⱥ�˳����______���ڰ���ȼ�յĻ�ѧ����ʽ��______��
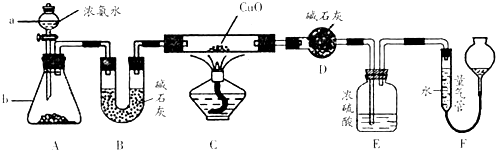
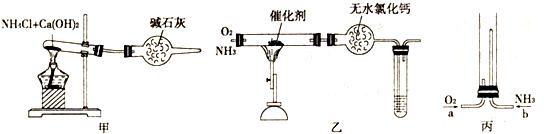
��1��ͼ����ʵ������ȡ����������İ�����װ��ͼ��д�����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O���������Ȼ��Ʒ�Ӧ�����������Բ������Ȼ��ƴ����ʯ�ң�
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O�����ܣ�
��2��NH3�����O2�ڴ��������������·�����Ӧ��4NH3+5O2
4NO+6H2O��ͨ����ˮCaCl2��δ��Ӧ���NH3�����ɵ�H2O�����գ����ɵ�NO���Թ��ڱ�������O2����Ϊ����ɫ��NO2���壬����ʽΪ��2NO+O2�T2NO2��
�ʴ�Ϊ������ɫ��4NH3+5O2
4NO+6H2O��
��3������װ��C�϶˿��ڣ�Ҫ��ȼNH3������ͨ��O2����ͨ��NH3����Ϊ����ͨ��NH3��NH3�ڿ����в��ܵ�ȼ��NH3�ݳ��������Ⱦ��NH3�ڴ�����ȼ�յĻ�ѧ����ʽΪ��4NH3+3O2
2N2+6H2O��O2�û�N2����
�ʴ�Ϊ����ͨO2����ͨNH3������ͨNH3�������ڿ����в���ȼ�գ��ݳ���ɻ�����Ⱦ��4NH3+3O2
2N2+6H2O��
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��2��NH3�����O2�ڴ��������������·�����Ӧ��4NH3+5O2
| ||
| �� |
�ʴ�Ϊ������ɫ��4NH3+5O2
| ||
| �� |
��3������װ��C�϶˿��ڣ�Ҫ��ȼNH3������ͨ��O2����ͨ��NH3����Ϊ����ͨ��NH3��NH3�ڿ����в��ܵ�ȼ��NH3�ݳ��������Ⱦ��NH3�ڴ�����ȼ�յĻ�ѧ����ʽΪ��4NH3+3O2
| ||
�ʴ�Ϊ����ͨO2����ͨNH3������ͨNH3�������ڿ����в���ȼ�գ��ݳ���ɻ�����Ⱦ��4NH3+3O2
| ||

��ϰ��ϵ�д�
�����Ŀ