��Ŀ����
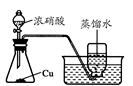
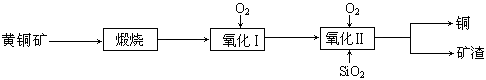
��14�֣���ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ���Ҫ�ɷ�ΪCuFeS2����������ʯ��Ϊ�ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
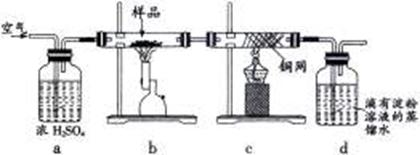
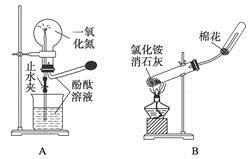
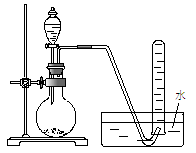
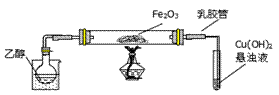
�õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��1/10������ƿ�У���0.05mol/L������Һ���еζ������ı�����Һ20.00mL����ش��������⣺
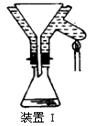
��1������Ʒ��ϸ���ٽ��з�Ӧ����Ŀ���� ��������ҺӦʢ���ڣ����ʽ������ʽ���� �ζ����С�
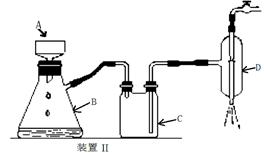
��2��װ��a��������
������ţ���
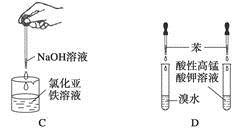
��3����ȥ��cװ�ã���ʹ�ⶨ������ƫ�͡�����ƫ�ߡ�����Ӱ�족�� ��д��Ӱ��ⶨ����Ļ�ѧ����ʽ�� ��
��4��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ���� ��
��5��ͨ�������֪���û�ͭ��Ĵ���Ϊ ��

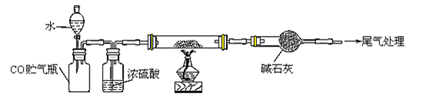
�õ�����ƽ��ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��1/10������ƿ�У���0.05mol/L������Һ���еζ������ı�����Һ20.00mL����ش��������⣺
��1������Ʒ��ϸ���ٽ��з�Ӧ����Ŀ���� ��������ҺӦʢ���ڣ����ʽ������ʽ���� �ζ����С�
��2��װ��a��������
������ţ���
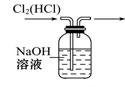
| A����ȥ�����еĶ�����̼ | B����ȥ�����е�ˮ���� |
| C�������������� | D�������ڹ۲졢���ƿ������� |
��4��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ���� ��
��5��ͨ�������֪���û�ͭ��Ĵ���Ϊ ��
��14�֣�
��1��ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ�2�֣���ʽ��2�֣�
��2��B D��2�֣�
��3��ƫ�ͣ�2�֣� ��2�֣�
��2�֣�
��4��ʹ��Ӧ���ɵ�����ȫ������dװ���У�ʹ�ⶨ�����ȷ��2�֣� ��5��80%��2�֣�
��1��ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ�2�֣���ʽ��2�֣�
��2��B D��2�֣�
��3��ƫ�ͣ�2�֣�
 ��2�֣�
��2�֣���4��ʹ��Ӧ���ɵ�����ȫ������dװ���У�ʹ�ⶨ�����ȷ��2�֣� ��5��80%��2�֣�
�����������1������Ʒ��ϸ���ٷ�Ӧ�����������ı������Ŀ����ʹԭ�ϳ�ַ�Ӧ���ӿ췴Ӧ���ʣ���ҺΪI2��Һ�����������ԣ��������ܣ������ü�ʽ�ζ���ʢ�ţ�Ӧѡ����ʽ�ζ��ܡ�
��2��װ��a�е�Ũ����������տ����е�ˮ������ͬʱ����ð�������ݵ����������������ͨ�������ʴ�Ϊ��BD��
��3��ȥ��cװ�ã������ж���������ˮ��Һ�л��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2SO2+O2+H2O=2H2SO4���ⶨ���ƫ�͡�
��4����ͭ�����ȷֽ����ɶ��������һϵ�в���ֽ���Ϻ���Ȼͨ����������Խ������Ķ�������ȫ���ų�ȥ������dװ���У�ʹ�����ȷ��
��5������������ԭ��Ӧ�е�ʧ����������Ⱥ�SԪ���غ�ɵõ���2I2��2SO2��CuFeS2�����ĵ�0.05mol/L������Һ20.00mLʱ�������ĵĵⵥ�ʵ���Ϊ��0.05mol/L��0.02L=0.0010mol�����Ի�ͭ��������ǣ�0.5��0.0010mol��184g/mol��10=0.92g�������䴿���ǣ�0.92g��1.15g��100%=80%��

��ϰ��ϵ�д�
�����Ŀ
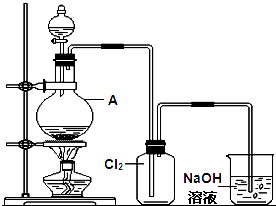





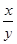 ��_____��
��_____��