��Ŀ����
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ���ش��������⣺
��1��S��N��O�ĵ�һ�������ɴ�С��˳��Ϊ ��
��2��Ѫ�쵰���к���Fe2����CO����Ѫ�쵰��ϳ��ȶ���������ʹ���ж���
��д���������ӵĻ�̬�����Ų�ʽ ��
��CO���Ժͺܶ���ɽ����γ�����CO������Cԭ������һ�Թ¶Ե��ӣ�C��Oԭ�Ӷ�����8�����ȶ��ṹ��CO������ԭ�ӵ��ӻ�����Ϊ �ӻ���CO�ж��ֵȵ����壬���г���������Ϊ ��
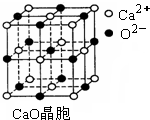
��3��SO2��һ�ִ�����Ⱦ�Ϊ����SO2��Ⱦ���������糧������ ����ȼú�м���CaO�ԡ�����CaO������ͼ��ʾ������Ca2+����λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3 401kJ/mol��NaCl��786kJ/mol��CaO������۵� NaCl������۵㣨����ڡ��������ڡ����ڡ�����

��4����������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��ԽΪ��������ע����ȩ��������Ҫ������Ⱦ��֮һ����е��ǣ�19.5�棩����ȩ�ǡ��پơ��е���Ҫ�к����ʣ���е���64.65�棩��1mol��ȩ������![]() ������ĿΪ ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���� ��
������ĿΪ ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���� ��
��1��N��O��S��2�֣�
����2����1s22s22p63s23p63d6 ��2�֣�
��sp ��2�֣�
N2��CN�� ��2�֣�
��3��6�������ڣ�2�֣�
����4��3NA�����״����Ӽ�������������ȩû�� ��2�֣�