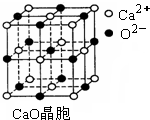
��Ŀ����
���������ߵ�ֱ��Ӱ�����������������������������ձ����п�����Ⱦָ������Ŀ��SO2�� CO��NOx��O3�Ϳ����������ȡ�����˵������ȷ���� �� ��
A�������еij�����Ե����кܺõı������ã����Դ����к��д���O3����������
B���ڴ��������£�CO��NOx��ת��Ϊ������
C��Ѫ�쵰���к���Fe2+��NO��CO������Ѫ�쵰��ϳ��ȶ����ʶ�ʹ���ж�
D��SO2��NOx�Ĵ����ŷŻᵼ������IJ���
���𰸡�
A
����������

��ϰ��ϵ�д�
�����Ŀ