��Ŀ����
��13�֣�
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
��1��S��N��O�ĵĵ�һ�������ɴ�С��˳��Ϊ ��
��2��SO2��CO��NO2��O3�����¾�Ϊ���壬��̬ʱ������ ���塣
��3����������������������ߣ����ڵĻ�����ȫ��ʳƷ��ȫԽ��ԽΪ��������ע����ȩ��HCHO����������Ҫ������Ⱦ��֮һ����е��ǨC19.5 �棩���״���CH3OH���ǡ��پơ��е���Ҫ�к����ʣ���е���64.65 �棩����ȩ������Cԭ�Ӳ�ȡ �ӻ������ʽ���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ�__________ ��
��4��CuCl��������Һ�ܹ���CO������Ӧ��CuCl+CO+H2O=Cu(CO)Cl��H2O���÷�Ӧ�����ڲⶨ������CO������
��д��ͭԭ�ӵĻ�̬�����Ų�ʽ ��
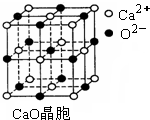
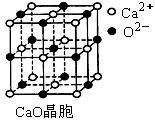
��CuCl�ľ���ṹ����ͼ����ʾ����ͬһ��Cl���������������Cu���� ����

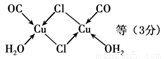
��Cu(CO)Cl��H2O�Ľṹ����ͼ����ʾ��ͼ�б�ʾ��8���ǹ��ۼ�������6������λ��������ͼ���ü�ͷ��ʾ����
��13�֣�
��1��N>O>S��2�֣�
��2�����ӣ�1�֣�
��3��sp2 (1��) �״����Ӽ�������������ȩû�У�2�֣�
��4����1s22s22p63s23p63d104s1����[Ar] 3d104s1�� ��2�֣�
��4��2�֣�
��
��������

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�