��Ŀ����
10��������A��CԪ�ص���������Ϊ64.86%��HԪ�ص���������Ϊ13.51%������ΪOԪ�أ���1��A�ķ���ʽ��C4H10O��
��2��A���Է������±仯��
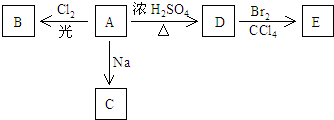
��A�����еĹ������������ǻ���
��Aֻ��һ��һ��ȡ����B��д����Aת��ΪB�Ļ�ѧ����ʽ��CH3��3C-OH+Cl2$\stackrel{����}{��}$��CH3��2C��OH��CH2Cl��
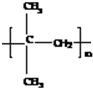
��D�����Ӿ۷�Ӧ������Ľṹ��ʽ��
 ��
����X��D��ͬϵ���з�������С�Ļ����X�ĵ���ʽ��
 ��
����B��C��һ�������·���ȡ����Ӧ������NaCl��Y��Y�Ľṹ��ʽ��C��CH3��3OC��CH3��2OH��
��3��A��ͬ���칹��FҲ�����п�ͼ��A�ĸ��ֱ仯���ֱ�����B�䡢C�䡢D�䡢E�䣮 E�䷢����ȥ��Ӧ���ɵĻ�����Z������̼ԭ����һ��ֱ���ϣ�
��F�Ľṹ��ʽ��CH3CH��OH��CH2CH3��
��E��ת��ΪZ�Ļ�ѧ����ʽ��CH3CHBrCHBrCH3+2NaOH$��_{��}^{�Ҵ�}$CH3C��CCH3+2NaBr+2H2O��
�ۺ�18O��F�����ᷴӦ�Ļ�ѧ����ʽ��CH3COOH+CH3CH��18OH��CH2CH3$?_{��}^{Ũ����}$CH3COOCH��CH3��CH2CH3+H2O����
���� ��1��������A����Է�������Ϊ74��������A��CԪ�ص���������Ϊ64.86%��HԪ�ص���������Ϊ13.51%������ΪOԪ�أ�A��C��H��OԪ��ԭ�Ӹ���֮��=$\frac{64.86%}{12}$��$\frac{13.51%}{1}$��$\frac{1-64.86%-13.51%}{16}$=4��10��1��A�����ʽΪC4H10O����ΪA����Է�������Ϊ74����A�ķ���ʽΪC4H10O��
��2��A�ܺ�Na��Ӧ����A���д��ǻ���Aֻ��һ��һ��ȡ����B����A�ṹ��ʽΪ��CH3��3COH��A����ȡ����Ӧ����B��B�ṹ��ʽΪ��CH3��2C��OH��CH2Cl��A���Ʒ�Ӧ����C��C�ṹ��ʽΪ��CH3��3CONa��A������ȥ��Ӧ����D��DΪ��CH3��2C=CH2��D���巢���ӳɷ�Ӧ����E��E�ṹ��ʽΪ��CH3��2CBrCH2Br��
��3��A��ͬ���칹��FҲ�����п�ͼ��A�ĸ��ֱ仯���ֱ�����B�䡢C�䡢D�䡢E�䣻 E�䷢����ȥ��Ӧ���ɵĻ�����Z������̼ԭ����һ��ֱ���ϣ���Z�ṹ��ʽΪCH3C��CCH3��E��ṹ��ʽΪCH3CHBrCHBrCH3��F�ṹ��ʽΪCH3CH��OH��CH2CH3��C��ṹ��ʽΪCH3CH��ONa��CH2CH3���ݴ˷������
��� �⣺��1��������A����Է�������Ϊ74��������A��CԪ�ص���������Ϊ64.86%��HԪ�ص���������Ϊ13.51%������ΪOԪ�أ�A��C��H��OԪ��ԭ�Ӹ���֮��=$\frac{64.86%}{12}$��$\frac{13.51%}{1}$��$\frac{1-64.86%-13.51%}{16}$=4��10��1��A�����ʽΪC4H10O����ΪA����Է�������Ϊ74����A�ķ���ʽΪC4H10O��
�ʴ�Ϊ��C4H10O��
��2��A�ܺ�Na��Ӧ����A���д��ǻ���Aֻ��һ��һ��ȡ����B����A�ṹ��ʽΪ��CH3��3COH��A����ȡ����Ӧ����B��B�ṹ��ʽΪ��CH3��2C��OH��CH2Cl��A���Ʒ�Ӧ����C��C�ṹ��ʽΪ��CH3��3CONa��A������ȥ��Ӧ����D��DΪ��CH3��2C=CH2��D���巢���ӳɷ�Ӧ����E��E�ṹ��ʽΪ��CH3��2CBrCH2Br��
��AΪ��CH3��3COH�����������ǻ����ʴ�Ϊ���ǻ���
��AΪ��CH3��3COH��BΪ��CH3��2C��OH��CH2Cl���ڹ��������£�A����������ȡ����Ӧ����B����Ӧ����ʽΪ ��CH3��3C-OH+Cl2$\stackrel{����}{��}$��CH3��2C��OH��CH2Cl��
�ʴ�Ϊ����CH3��3C-OH+Cl2$\stackrel{����}{��}$��CH3��2C��OH��CH2Cl��
��DΪ��CH3��2C=CH2��D�ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ���������ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��DΪ��CH3��2C=CH2��X��D��ͬϵ���з�������С�Ļ����Ϊ��ϩ����X�ĵ���ʽ��
���ʴ�Ϊ�� ��
��
��B��C��һ�������·���ȡ����Ӧ������NaCl��Y��Y�ṹ��ʽΪC��CH3��3OC��CH3��2OH��
�ʴ�Ϊ��C��CH3��3OC��CH3��2OH��
��3��A��ͬ���칹��FҲ�����п�ͼ��A�ĸ��ֱ仯���ֱ�����B�䡢C�䡢D�䡢E�䣻 E�䷢����ȥ��Ӧ���ɵĻ�����Z������̼ԭ����һ��ֱ���ϣ���Z�ṹ��ʽΪCH3C��CCH3��E��ṹ��ʽΪCH3CHBrCHBrCH3��F�ṹ��ʽΪCH3CH��OH��CH2CH3��C��ṹ��ʽΪCH3CH��ONa��CH2CH3��
��ͨ�����Ϸ���֪��F�ṹ��ʽΪCH3CH��OH��CH2CH3���ʴ�Ϊ��CH3CH��OH��CH2CH3��
��E�䷢����ȥ��Ӧ���ɵĻ�����Z������̼ԭ����һ��ֱ���ϣ���Z�ṹ��ʽΪCH3C��CCH3��E��ṹ��ʽΪCH3CHBrCHBrCH3��E�䷢����ȥ��Ӧ���ɵĻ�����Z�ķ�Ӧ����ʽΪCH3CHBrCHBrCH3+2NaOH$��_{��}^{�Ҵ�}$CH3C��CCH3+2NaBr+2H2O��
�ʴ�Ϊ��CH3CHBrCHBrCH3+2NaOH$��_{��}^{�Ҵ�}$CH3C��CCH3+2NaBr+2H2O��
�ۺ�18O��F�����ᷴӦ�Ļ�ѧ����ʽ��CH3COOH+CH3CH��18OH��CH2CH3$?_{��}^{Ũ����}$ CH3COOCH��CH3��CH2CH3+H2O���ʴ�Ϊ��CH3COOH+CH3CH��18OH��CH2CH3$?_{��}^{Ũ����}$ CH3COOCH��CH3��CH2CH3+H2O��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ���������ȷ�����ż������ʹ�ϵ����Ӧ�����ǽⱾ��ؼ���ע��������Ӧ�жϼ��ͳɼ�λ�ã�Ϊ�״��㣮

| A�� | C14H18O5 | B�� | C14H16O4 | C�� | C14H22O5 | D�� | C14H10O5 |
| A�� | Na����ˮ�У��������壺2Na+2H2O�T2Na++2OH-+H2�� | |
| B�� | FeCl3��Һ��ʴӡˢ��·�壺2Fe3++Cu�T2Fe2++Cu2+ | |
| C�� | ͭ�����ữ��H2O2��Һ��Cu+2H++H2O2�TCu2++2H2O | |
| D�� | AlƬ������NaOH��Һ��Ӧ���������壺2Al+2OH-+2H2O�T2Al��OH��3+3H2�� |
| A�� | NH4CNO | B�� | CH4 | C�� | 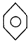 | D�� | CH3CH2OH |
| A�� | ���ܷ�����ȥ��Ӧ | B�� | ������Ϊͪ | ||
| C�� | ����Na��Ӧ | D�� | ���ܷ���ȡ����Ӧ |
| A�� | �����в����ܺ������Ӽ� | B�� | ���ۻ������в����ܺ������Ӽ� | ||
| C�� | ֻ�зǽ���ԭ�Ӽ�����γɹ��ۼ� | D�� | �ǽ��������в�һ�����й��ۼ� |
| A�� | CH4 | B�� | C2H6 | C�� | C3H8 | D�� | C4H10 |