��Ŀ����
20�� ����ͼ��ʾװ���У��Թ�A��B�еĵ缫Ϊ��Ķ��Ե缫��C��DΪ�������м��ڱ�Na2SO4��Һ��ʪ����ֽ���ϣ���ֽ�����в�����KMnO4Һ�Σ���Դ��a��b����������A��B�г���KOH��Һ������ʢKOH��Һ��ˮ���У��Ͽ�K1���պ�K2��K3��ֱͨ���磬ʵ��������ͼ��ʾ����ش���������
����ͼ��ʾװ���У��Թ�A��B�еĵ缫Ϊ��Ķ��Ե缫��C��DΪ�������м��ڱ�Na2SO4��Һ��ʪ����ֽ���ϣ���ֽ�����в�����KMnO4Һ�Σ���Դ��a��b����������A��B�г���KOH��Һ������ʢKOH��Һ��ˮ���У��Ͽ�K1���պ�K2��K3��ֱͨ���磬ʵ��������ͼ��ʾ����ش�����������1�������Դ������������aΪ������bΪ������
��2����ʪ��Na2SO4��ֽ�����в�KMnO4Һ�δ�����������Ϊ��ɫ��D�����ƶ����������������
��3�������һ��ʱ���A��B�о��������Χ�缫����ʱ�ж�K2��K3���պ�K1�����������ָ���ǣ���ǡ���ƫת��
��4������������ָ�뷢��ƫת����д���йصĵ缫��Ӧʽ��A��O2+2H2O+4e-�T4OH-
B��2H2+4OH--4e-�T4H2O����ָ�벻����ƫת������ⲻ��Ҫ�ش𣩣���ָ�벻����ƫת����˵�����ɶ��ࣨ��ָ�뷢��ƫת������ⲻ��Ҫ�ش𣩣�
���� A��B�е缫Ϊ��Ķ��Ե缫��C��DΪ����ʪ��Na2SO4��ֽ���ϵIJ��У���Դ��a��b����������A��B�г���KOH��Һ������KOH��Һ��ˮ���У��ж�K1���պ�K2��K3��ֱͨ���磬AB��CD���ǵ��ԭ����Ӧ�ã�����AB����������仯������������ˮ��A�˲������������ص�������B����������Ϊ���ص���������aΪ��Դ�ĸ�����bΪ��Դ������
��1������ԭ��صĹ���ԭ����д�缫��Ӧʽ��
��2�����ж�C��D �ĵ缫���ƣ��жϵ��ʱ��Һ�����ӵ��ƶ�����
��3�������һ��ʱ�䣬A��B�о��������Χ�缫����ʱ�ж�K2��K3���պ�K1����������ָ��ƫת��˵��AB���γɵ�����ȼ�ϵ�أ�����ԭ���ԭ�������жϣ�
��4������ԭ�����ȼ�ϵ�صĹ���ԭ���������ش�
��� �⣺��1���ж�K1���ϱ�K2��K3ֱͨ���磬�缫A��B������������Һ���ɵ��أ��������ӵķŵ�˳����Һ�������ӡ����������ӷŵ磬�ֱ����������������������������������Ϊ2��1��ͨ��ͼ��֪��B�������������A�������������2��������B���ϵ�������A���ϵõ�����������B����������A������������a�Ǹ�����b��������
�ʴ�Ϊ��������������
��2�����������Ƶ���ֽ�͵缫C��D���ԴҲ�����˵��أ���Ϊa�Ǹ�����b������������C��������D���������������Һ�е������Ӽ������������ƶ��������Ӹ�����������������ƶ�������D������ɫ���������Һ�������Ӻ����������ӷŵ磬�����������϶��õ����壮
�ʴ�Ϊ����ɫ��D�����ƶ������������������
��3���ж�K2��K3���ϱ�K1�����һ��ʱ���A��B�о��������Χ�缫����װ�ù�������ȼ��ԭ��أ������е���ͨ������������ָ���ƶ���
�ʴ�Ϊ���ǣ�
��4�������һ��ʱ���A��B�о��������Χ�缫����ʱ�ж�K2��K3���պ�K1����������ȼ�ϵ�أ���ȼ�ϵ���У�ȼ������BΪ����������ʽΪ2H2+4OH--4e-=4H2O��
����A������������ʽΪO2+2H2O+4e-=4OH-��
�ʴ�Ϊ��O2+2H2O+4e-=4OH-��2H2+4OH--4e-=4H2O��
���� ���⿼����ԭ��ء����ع���ԭ����д�缫��ӦʽҪע���ϵ������Һ��д������������Һ��ͬ����Ȼԭ����ͬ���缫��ӦʽҲ��ͬ��������ȼ�ϵ�أ��������Ϊ����缫��Ӧʽ�Ͳ�ͬ��

| A�� | ���������� | B�� | Һ������Ȼ�̼ | C�� | ��ȩ��ˮ | D�� | �屽��ˮ |
| A�� | �٢ڢۢ� | B�� | �ۢ٢ڢ� | C�� | �ܢڢۢ� | D�� | �ܢڢ٢� |
| A�� | ������ | B�� | ��ά�� | C�� | ��֬ | D�� | ������ |
| A�� | ������CS2���۵�112.8�棬�е�444.6�� | |
| B�� | �۵�1003.1�棬Һ̬ʱ���磬ˮ��Һ���� | |
| C�� | �۵�1070�棬������ˮ��ˮ��Һ�ܵ��� | |
| D�� | �۵�97.81�棬���������磬�ܶ�0.97 g•cm-3 |
| ��� | �� | �� | �� |
| ʵ�� |  |  | 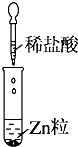 |
| ���� | û�����Ա仯����Һ��Ϊ��ɫ | �г������ɣ���ҺΪ��ɫ | ����ɫ����ų� |
| A�� | ���������Ա仯��˵������Һ����Ӧ | |
| B�� | ���еİ�ɫ����ΪBaSO4 | |
| C�� | ���е����ӷ���ʽΪ2H++Zn�TZn2++H2�� | |
| D�� | ���з����ķ�ӦҲ��������ԭ��Ӧ |
| A�� | ̼������ڴ�����Һ�У�CaCO3+2H+�TCa2++CO2��+H2O | |
| B�� | �Ȼ�þ��Һ�Ͱ�ˮ��ϣ�Mg2++2OH-�TMg��OH��2 | |
| C�� | ����ϡ���ᷴӦ��Fe+2H+�TFe2++H2�� | |
| D�� | ��Ƭ������������Һ�� Al+3Ag+�TAl3++3Ag |
| A�� | HClO�ĵ���ʽΪ 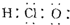 | |
| B�� | H2S�ĵ���ʽ�ɱ�ʾΪ | |
| C�� | �õ���ʽ��ʾNa2O���γɹ���Ϊ2Na��+ ��2Na+ ��2Na+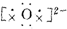 | |
| D�� | MgCl2�ĵ���ʽΪ Mg2+ Mg2+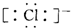 |