��Ŀ����
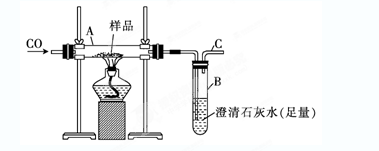
��16�֣�. ͭ����������֪���Ľ���֮һ��ʵ���ҿ���C��H2��ԭCuO��ȡ������Cu����ҵ����Ҫ�ûӻ�ͭ��������Cu��
��. ʵ��֤����C��ԭCuOʱ�ȿ�������Cu��Ҳ��������Cu2O����ʹ��C����ʱ��ʵ����CuOҲ���ܲ���δ����ԭ��Ϊ�˲ⶨij��ʵ��������ɣ�ȡ1.2 g C��8.0 g CuO��ϼ��ȣ�����Ӧ���ɵ�����ͨ�������ij���ʯ��ˮ��������һ��ʱ���ֹͣ���ȣ����ռ���560 mL���壨�Ѿ�����ɱ�״��������ó���������Ϊ2.5 g����
��1������ʵ����C �����ȫ������ȫ�����μӷ�Ӧ��ʵ�����ռ�������
���� ����д��ѧʽ����������������Ļ�ѧ����ʽΪ�� ��
��2����Ӧ��õ��Ĺ�������������Ϊ �����к�������������ʵ���Ϊ mol��
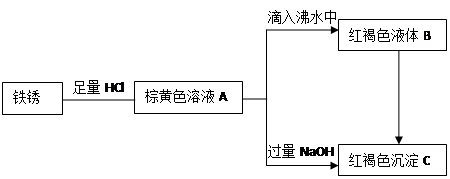
��. ��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ���� 2��12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05mol/L��NaOH��Һ������һ����Һ�еμ�0��600mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��
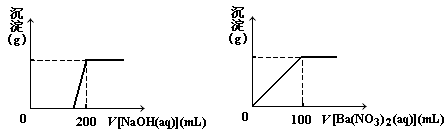
��1�� ��ͨ������ȷ��m��ֵ��
��2�� X��Ħ������Ϊ368 g/mol����ȷ��X�Ļ�ѧʽ��
��. ʵ��֤����C��ԭCuOʱ�ȿ�������Cu��Ҳ��������Cu2O����ʹ��C����ʱ��ʵ����CuOҲ���ܲ���δ����ԭ��Ϊ�˲ⶨij��ʵ��������ɣ�ȡ1.2 g C��8.0 g CuO��ϼ��ȣ�����Ӧ���ɵ�����ͨ�������ij���ʯ��ˮ��������һ��ʱ���ֹͣ���ȣ����ռ���560 mL���壨�Ѿ�����ɱ�״��������ó���������Ϊ2.5 g����
��1������ʵ����C �����ȫ������ȫ�����μӷ�Ӧ��ʵ�����ռ�������
���� ����д��ѧʽ����������������Ļ�ѧ����ʽΪ�� ��
��2����Ӧ��õ��Ĺ�������������Ϊ �����к�������������ʵ���Ϊ mol��
��. ��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ���� 2��12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05mol/L��NaOH��Һ������һ����Һ�еμ�0��600mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��

��1�� ��ͨ������ȷ��m��ֵ��
��2�� X��Ħ������Ϊ368 g/mol����ȷ��X�Ļ�ѧʽ��
��Ų���ȫ(1��)��CO(1��)��CO2+C����2CO �� CuO+C����Cu+CO�� (1��)��
�� 7.4g��(1��)�� 0.025 mol(1��)
�� �⣺�����⣬m g X�У�n(Cu)��n(Fe)��1��1
2n(Cu2��) ��3n(Fe3��) ��6��05mol��L��1��0��2L��2��1 mol��L��1��2��12L
�ʣ�n (Cu) �� n (Fe) �� 0��06mol
�֣�n (S) �� 0��6mol��L��1��0��1L��2 �� 0��12mol
��ˣ�m g �� m(Cu) �� m(Fe) �� m(S)
�� 0��06mol��64g��mol��1��0��06mol��56g��mol��1��0��12mol��32g��mol��1�� 11��04g
��m ��ֵΪ11��04 ��3�֣�
�� �⣺��X�Ļ�ѧʽΪ(CuFeS2)n����
(64��56��32��2)��n �� 368 n �� 2��X�Ļ�ѧʽΪCu2Fe2S4 ��3�֣�
�� 7.4g��(1��)�� 0.025 mol(1��)
�� �⣺�����⣬m g X�У�n(Cu)��n(Fe)��1��1
2n(Cu2��) ��3n(Fe3��) ��6��05mol��L��1��0��2L��2��1 mol��L��1��2��12L
�ʣ�n (Cu) �� n (Fe) �� 0��06mol
�֣�n (S) �� 0��6mol��L��1��0��1L��2 �� 0��12mol
��ˣ�m g �� m(Cu) �� m(Fe) �� m(S)
�� 0��06mol��64g��mol��1��0��06mol��56g��mol��1��0��12mol��32g��mol��1�� 11��04g
��m ��ֵΪ11��04 ��3�֣�
�� �⣺��X�Ļ�ѧʽΪ(CuFeS2)n����
(64��56��32��2)��n �� 368 n �� 2��X�Ļ�ѧʽΪCu2Fe2S4 ��3�֣�
��1��560ml������CO�����ʵ�����0.025mol������CO�ķ���ʽ��CO2+C����2CO �� CuO+C����Cu+CO����������̼��ƣ����ʵ�����0.025mol�������ɵ�CO2Ҳ��0.025mol������̼�����ʵ�����0.1mol������ʵ����̼û����ȫ�μӷ�Ӧ��
��2����̼ԭ�ӽ�ϵ���ԭ�ӵ����ʵ�����0.025mol��0.025mol��2��0.075mol��������12.g���������չ����������8g��1.2g��1.2g��0.6g��7.4g��ԭ����ͭ����ԭ�ӵ����ʵ�����0.1mol������ʣ����ԭ�ӵ����ʵ�����0.1mol��0.075mol��0.025mol����˺�������������ʵ�����0.025mol��
������ݷ���ʽ���й�ͼ����е��йؼ��㡣
��2����̼ԭ�ӽ�ϵ���ԭ�ӵ����ʵ�����0.025mol��0.025mol��2��0.075mol��������12.g���������չ����������8g��1.2g��1.2g��0.6g��7.4g��ԭ����ͭ����ԭ�ӵ����ʵ�����0.1mol������ʣ����ԭ�ӵ����ʵ�����0.1mol��0.075mol��0.025mol����˺�������������ʵ�����0.025mol��
������ݷ���ʽ���й�ͼ����е��йؼ��㡣

��ϰ��ϵ�д�
�����Ŀ