��Ŀ����
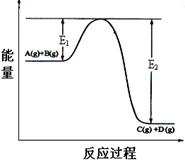
(1)��ӦA(g)+B(s) C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
������___ ��������ѹǿ____ �������������ݻ�____ ��
�ܼ���A___ �� �ݼ���B____ �� ��������____ ��
��2����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________________��
��3���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪��C3H8(g) CH4(g)��HC
CH4(g)��HC CH(g)��H2(g) ��H1="156.6" kJ��mol��1
CH(g)��H2(g) ��H1="156.6" kJ��mol��1
CH3CH CH2(g)
CH2(g) CH4(g)��HC
CH4(g)��HC CH(g ) ��H2="32.4" kJ��mol��1
CH(g ) ��H2="32.4" kJ��mol��1
����ͬ�����£���ӦC3H8(g) CH3CH
CH3CH CH2(g)��H2(g) �ġ�H= kJ��mol��1��
CH2(g)��H2(g) �ġ�H= kJ��mol��1��
��4���±��е����ݱ�ʾ�ƻ�1 mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol��1)��
���ݼ������ݼ������·�Ӧ�ķ�Ӧ�ȡ�H��
CH4��g��+4F2��g����CF4��g��+4HF��g�� ��H=______________________
 C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ�
C(g)����H��0����������������ʱ���ı�����һ��������������C�����ʣ� ������___ ��������ѹǿ____ �������������ݻ�____ ��
�ܼ���A___ �� �ݼ���B____ �� ��������____ ��
��2����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________________��
��3���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪��C3H8(g)
 CH4(g)��HC
CH4(g)��HC CH(g)��H2(g) ��H1="156.6" kJ��mol��1
CH(g)��H2(g) ��H1="156.6" kJ��mol��1CH3CH
 CH2(g)
CH2(g) CH4(g)��HC
CH4(g)��HC CH(g ) ��H2="32.4" kJ��mol��1
CH(g ) ��H2="32.4" kJ��mol��1����ͬ�����£���ӦC3H8(g)
 CH3CH
CH3CH CH2(g)��H2(g) �ġ�H= kJ��mol��1��
CH2(g)��H2(g) �ġ�H= kJ��mol��1����4���±��е����ݱ�ʾ�ƻ�1 mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol��1)��
| ��ѧ�� | C��H | C��F | H��F | F��F |
| ���� | 414 | 489 | 565 | 158 |
CH4��g��+4F2��g����CF4��g��+4HF��g�� ��H=______________________
��1���ӿ졢�ӿ졢�������ӿ졢���䡢�ӿ� ��1��/ÿ�գ�
��2��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H���C889.6kJ��mol��1��4�֣�
��3��124.2��3�֣� ��4����1928 KJ/mol��3�֣�
��2��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H���C889.6kJ��mol��1��4�֣�
��3��124.2��3�֣� ��4����1928 KJ/mol��3�֣�
��1��������������Է�Ӧ���ʵ�Ӱ�졣����Ӧ���Ũ�ȡ������¶ȡ�����ѹǿ��ʹ�������������ܼӿ췴Ӧ���ʡ������������ݻ���ѹǿ��С����Ӧ���ʽ��͡�B�ǹ��壬����B����������Ӧ���ʲ��䡣
��2��ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������������֪��1mol������ȫȼ�շų���������55.6kJ��16��889.6 kJ�����Լ����ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H���C889.6kJ��mol��1��
��3�������˹���ɵ�Ӧ�á���������٣��ڼ��õ�C3H8(g) CH3CH
CH3CH CH2(g)��H2(g)�����Ը÷�Ӧ�ķ�Ӧ����156.6 kJ��mol��1��32.4 kJ��mol��1��124.2 kJ��mol��1��
CH2(g)��H2(g)�����Ը÷�Ӧ�ķ�Ӧ����156.6 kJ��mol��1��32.4 kJ��mol��1��124.2 kJ��mol��1��
��4����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����414 kJ��mol��1��4��158 kJ��mol��1��4��489 kJ��mol��1��4��565 kJ��mol��1��4����1928 KJ/mol��
��2��ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������������֪��1mol������ȫȼ�շų���������55.6kJ��16��889.6 kJ�����Լ����ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H���C889.6kJ��mol��1��
��3�������˹���ɵ�Ӧ�á���������٣��ڼ��õ�C3H8(g)
 CH3CH
CH3CH CH2(g)��H2(g)�����Ը÷�Ӧ�ķ�Ӧ����156.6 kJ��mol��1��32.4 kJ��mol��1��124.2 kJ��mol��1��
CH2(g)��H2(g)�����Ը÷�Ӧ�ķ�Ӧ����156.6 kJ��mol��1��32.4 kJ��mol��1��124.2 kJ��mol��1����4����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����414 kJ��mol��1��4��158 kJ��mol��1��4��489 kJ��mol��1��4��565 kJ��mol��1��4����1928 KJ/mol��

��ϰ��ϵ�д�
�����Ŀ
 O2(g) == H2O(l) ��H3����285.8kJ��mol-1
O2(g) == H2O(l) ��H3����285.8kJ��mol-1 O2(g)==H2O(g);��H3�����H1����H2�͡�H3�Ĺ�ϵ��
O2(g)==H2O(g);��H3�����H1����H2�͡�H3�Ĺ�ϵ��  2SO3 (g)����H����QkJ��mol��1(Q��0)����2molSO2(g)������O2����һ�ܱ������У���ַ�Ӧ��ų�������һ��С��QkJ
2SO3 (g)����H����QkJ��mol��1(Q��0)����2molSO2(g)������O2����һ�ܱ������У���ַ�Ӧ��ų�������һ��С��QkJ C(g)+D(g) ��H =" Q" kJ/mol
C(g)+D(g) ��H =" Q" kJ/mol