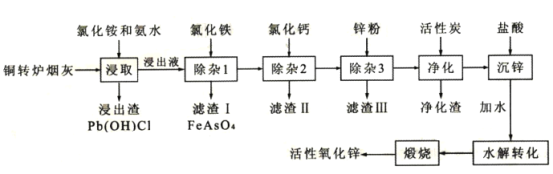
��Ŀ����
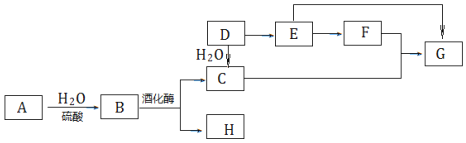
����Ŀ��A����Ȼ�л��߷��ӻ����D��һ����Ҫ�Ļ���ԭ�ϣ�����ͬ�����£�G�������ܶ���E��2��������֮���ת����ϵ��ͼ��
(1)���F����������������______��
(2)�������Ϊ������E����������G���÷�Ӧ�Ļ�ѧ����ʽ��______��
(3)����Aת����������B���ɣ����к�ˮ��Һ�����������Լ���______��
(4)����˵������ȷ����______��
A.����ת����ϵ���мӳɷ�Ӧ��ȡ����Ӧ��������Ӧ
B.�л���B��C��D��E����ʹ����KMnO4��Һ��ɫ
C.�л���C��F��ŨH2SO4�����¿����Ƶ�G���÷�Ӧ��ŨH2SO4�Ǵ�������ˮ��
D.ij��X����Է���������D��H֮�ͣ���X������ˮ�����ӳɷ�Ӧ��
���𰸡��Ȼ� 2CH3CHO![]() CH3COOCH2CH3 ������Һ������Cu(OH)2����Һ D
CH3COOCH2CH3 ������Һ������Cu(OH)2����Һ D
��������
A��Ȼ�л��߷��ӻ������������������ˮ�����B��B���ھƻ�ø�����·ֽ⣬˵��AΪ���ۣ�BΪ�����ǣ�CΪ�Ҵ���HΪ������̼��D��һ����Ҫ�Ļ���ԭ�ϣ�����ˮ��Ӧ�����Ҵ���˵��DΪ��ϩ����Ͽ�ͼC+F��G����֪EΪCH3CHO��FΪCH3COOH��GΪCH3COOCH2CH3��G�������ܶ���E��2�������E��GΪ2CH3CHO![]() CH3COOCH2CH3�����������Ƿ����к���ȩ�������Ա�������Һ������Cu(OH)2����Һ������������ڣ����ø��ֲ�ͬ���ʵĽṹ�����ʷ����жϣ��Դ˽����⡣
CH3COOCH2CH3�����������Ƿ����к���ȩ�������Ա�������Һ������Cu(OH)2����Һ������������ڣ����ø��ֲ�ͬ���ʵĽṹ�����ʷ����жϣ��Դ˽����⡣
(1)������������֪FΪ���ᣬ�ṹ��ʽΪCH3COOH�����еĹ���������Ϊ�Ȼ���
(2)�������Ϊ������E�������γ�G����E��G�ķ���ʽΪ��2CH3CHO![]() CH3COOCH2CH3��
CH3COOCH2CH3��
(3)A�ǵ��ۣ���ϡ����������������ˮ�������B�������ǣ�Ҫ֤�������Ƿ����к���ȩ����Ӧ�����ü��к����ᣬʹ��Һ�Լ��ԣ�Ȼ�����ȩ������ǿ��ԭ�ԣ����������Ƶ�������Һ��ˮԡ���ȣ�ͨ��������Ӧ���飻Ҳ����ͨ���������Ƶ�Cu(OH)2����Һ��ͨ��������У��۲��Ƿ����ש��ɫ���������жϣ�
(4)A.����������ȡ����Ӧ��A��B��C+F��G���ӳɷ�Ӧ��D��C��E��G��������Ӧ��D��E��E��F��A��ȷ��
B.����B�������ǣ������к��ǻ���ȩ��������C���Ҵ��������ǻ�������D����ϩ�������к��в����͵�̼̼˫����E����ȩ������ȩ������Щ�����к��еĹ����Ŷ��ܱ�����KMnO4�������������ʹ����KMnO4��Һ��ɫ��B��ȷ��
C.C+F��G��������Ӧ��ŨH2SO4�Ǹ÷�Ӧ�Ĵ�������ˮ����C��ȷ��
D.����D����ϩCH2=CH2����Է���������28������H��CO2����Է���������44�����ߵ���Է��������ĺ͵���72��ij��X����Է���������D��H֮�ͣ��������ʽΪC5H12��C5H12���������������������ͼ�����������ˮ�����ӳɷ�Ӧ��D����
�ʺ���ѡ����D��

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�����Ŀ��ijѧϰС��ͨ������װ��̽��![]() ��
��![]() �ܷ�Ӧ����
�ܷ�Ӧ����![]() ��
��
ʵ��������������±���
ʵ���� | ���� | ���� |
ʵ��1 | ����ͼ��ʾ���� |
|
ʵ��2 | ��A�еĻ���ﻻΪ B����Һ��ΪKSCN��Һ�����ȡ� | A�в��ֹ����ܽ⣬���������ͻ�ɫ���壬B��KSCN��Һ��� |
�ش��������⣺
![]() ʵ��1��ʵ��2����������ԭ����__________________________________________��
ʵ��1��ʵ��2����������ԭ����__________________________________________��
![]() ʵ��2˵����ɫ�����к���___________
ʵ��2˵����ɫ�����к���___________![]() �ѧʽ
�ѧʽ![]() ��ʵ��1��
��ʵ��1��![]() ������Ҳ�����Ƿ�������һ�����ӷ�Ӧ���������ӷ���ʽΪ_________________________________________��
������Ҳ�����Ƿ�������һ�����ӷ�Ӧ���������ӷ���ʽΪ_________________________________________��
![]() Ϊ��һ��ȷ�ϻ�ɫ�����к���
Ϊ��һ��ȷ�ϻ�ɫ�����к���![]() ����ѧϰС���ʵ��1������ָĽ�������
����ѧϰС���ʵ��1������ָĽ�������
����1����A��B������ʢ��ij���Լ���ϴ��ƿC�����B����Һ�Ա�Ϊ��ɫ��
����2����B�е���KI��Һ�滻ΪNaBr��Һ�����B����Һ�ʳȺ�ɫ����δ���![]() ��
��
��1��C��ʢ�ŵ��Լ���______������2�м���![]() ���Լ�������______��ѡ��NaBr��Һ��������______________________________________________________��
���Լ�������______��ѡ��NaBr��Һ��������______________________________________________________��
![]() ʵ��1��ּ��Ⱥ�����Ӧ�б�������δ����������Ԫ������֮��Ϊ1��2����A�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________�������ӷ���ʽ��ʾβ�������ķ�ʽ___________________________________��
ʵ��1��ּ��Ⱥ�����Ӧ�б�������δ����������Ԫ������֮��Ϊ1��2����A�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________�������ӷ���ʽ��ʾβ�������ķ�ʽ___________________________________��
![]() ��ѧϰС����Ϊʵ��1����Һ���������ܻ�������һ��ԭ����____________________���������ʵ�鷽����֤�˲���___________________________________________________________________________________________________________________________________________________��
��ѧϰС����Ϊʵ��1����Һ���������ܻ�������һ��ԭ����____________________���������ʵ�鷽����֤�˲���___________________________________________________________________________________________________________________________________________________��
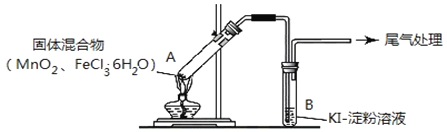
����Ŀ�����������(Na2S2O3)��һ�ֽⶾҩ�����ڷ�����顢����Ǧ����������ж����ٴ�����������ݡ���Ƥ�������Ȳ�֢.��������������Ի���Ի������ȶ�����������Һ�зֽ����S��SO2
ʵ��I��Na2S2O3���Ʊ�����ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��

(1)����a��������_______������b��������_______��b��������������Ϊ70%80%��H2SO4��Һ��Na2SO3���巴Ӧ�Ʊ�SO2��Ӧ�Ļ�ѧ����ʽΪ_______��c���Լ�Ϊ_______
(2)ʵ����Ҫ����SO2���������ʣ����Բ�ȡ�Ĵ�ʩ��_______ (д��һ��)
(3)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ����_______
ʵ���̽��Na2S2O3����������ӵ�������ԭ��Ӧ��
���ϣ�Fe3++3S2O32-Fe(S2O3)33-(�Ϻ�ɫ)
װ�� | �Լ�X | ʵ������ |
| Fe2(SO4)3��Һ | ��Ϻ���Һ�ȱ���Ϻ�ɫ��30s����Ϊ��ɫ |
(4)��������ʵ���������ж�����Fe3+��S2O32-��ԭΪFe2+��ͨ��_______(��������Լ�������)����һ��֤ʵ������Fe2+���ӻ�ѧ��Ӧ���ʺ�ƽ��ĽǶȽ���ʵ��������_______
ʵ��궨Na2S2O3��Һ��Ũ��
(5)��ȡһ�������IJ�Ʒ���Ƴ������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽȷ��ȡ������K2Cr2O7(Ħ������Ϊ294gmol-1)0.5880g��ƽ���ֳ�3�ݣ��ֱ����3����ƿ�У���ˮ�����Һ�������������KI���ữ���������з�Ӧ��6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������ӦI2+2S2O32- = 2I- + S4O62-���������� Na2S2O3��Һ��ƽ�����Ϊ25.00 mL�������궨�������������Һ��Ũ��Ϊ_______molL-1