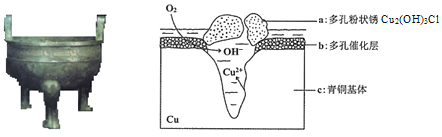
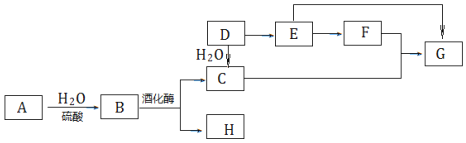
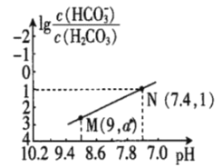
��Ŀ����
����Ŀ�������£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2 (g) �� 3 H2(g) ![]() 2NH3(g)��
2NH3(g)��
��1������Ӧijʱ��tʱ��n t (N2) = 13 mol��n t (NH3) = 6 mol����a =____mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������NH3�ĺ���(�������)Ϊ25%��ƽ��ʱNH3�����ʵ���_____��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȡ���ͬ����n(ʼ)��n(ƽ) =______��
��4��ԭ��������У�a��b =_____��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ���(N2)�æ�(H2)= ______��
��6��ƽ���������У�n(N2)��n(H2)��n(NH3) =______��
���𰸡�16 mol 8 mol 5��4 2��3 1��2 3��3��2
��������
��1�����ݻ�ѧ����ʽ����aֵ��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������������ʵ�����![]() 32mol������NH3�ĺ���(�������)Ϊ25%���㰱�������ʵ�����
32mol������NH3�ĺ���(�������)Ϊ25%���㰱�������ʵ�����
��3�����ò���������ԭ�����������ʵ�����
��4�����ݣ�1����֪a=16�����ݣ�3����֪ԭ�����������ʵ�����40mol��
��5�����á�����ʽ������N2��H2��ת����֮�ȣ�
��6�����ݡ�����ʽ���ж�ƽ���������и���������ʵ����ȣ�
��1������N2 (g)�� 3H2(g)![]() 2NH3(g)��Ӧ����֪����Ӧijʱ��tʱ��n (NH3) = 6 mol��������n (N2) =3 mol����ʼ����n (N2)= 13mol+ 3mol =16mol��
2NH3(g)��Ӧ����֪����Ӧijʱ��tʱ��n (NH3) = 6 mol��������n (N2) =3 mol����ʼ����n (N2)= 13mol+ 3mol =16mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������������ʵ�����![]() 32mol��NH3�ĺ���(�������)Ϊ25%�������������ʵ�����32mol��25%=8mol��
32mol��NH3�ĺ���(�������)Ϊ25%�������������ʵ�����32mol��25%=8mol��
��3���跴Ӧ���������ʵ�������n��

n=![]() 8mol������ԭ�����������ʵ�����32mol+8mol=40mol��ԭ���������ƽ��������������ʵ���֮��40:32=5:4��
8mol������ԭ�����������ʵ�����32mol+8mol=40mol��ԭ���������ƽ��������������ʵ���֮��40:32=5:4��
��4�����ݣ�1����֪a=16�����ݣ�3����֪ԭ�����������ʵ�����40mol������b=40mol-16mol=24mol��a��b =16:24=2:3��
��5��

N2��ת����Ϊ![]() ��H2��ת����Ϊ
��H2��ת����Ϊ![]() ��������(N2)����(H2)=1:2��
��������(N2)����(H2)=1:2��
��5������

ƽ���������У�n(N2)��n(H2)��n(NH3) =12:12:8=3:3:2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�