��Ŀ����
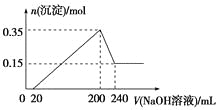
����Ŀ��I���ֱ�ȡ�����ʵ���Ũ�ȵ�����������Һ��100 mL����ͨ��һ������CO2�����ֱ��ȡ10 mL��Һ����������ε���0.2 mol��L��1�����ᣬ�ڱ�״���²���CO2�����(y��)����������������(x��)�Ĺ�ϵ����ͼ(��A��B�������)��
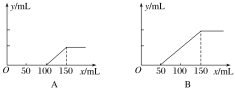
��1����A����£�������________________(�ѧʽ)�������ʵ���֮��Ϊ________��
��2��ԭ����������Һ�����ʵ���Ũ��Ϊ________________��
II���������������ֱ�Ͷ�뵽������������ʵ���Ũ�ȵ����������������Һ�У�����H2�����֮����__________________��
III����һ������Fe��FeO��Fe2O3�Ļ�����м���100 mLŨ��Ϊ2.0 mol��L��1�����ᣬǡ��ʹ�������ȫ�ܽ⣬�ų�224 mL������(��״��)��������Һ�м���KSCN��Һ����Ѫ��ɫ���֡�����������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ܵõ�����������________,����������_________________
���𰸡�NaOH��Na2CO3 1:1 3mol/L 1:3 5.6g ԭ���غ㣨�������غ㣩
��������
I����NaOH��Һ��ͨ��CO2������CO2��ͨ�룬������4�������NaOH��Na2CO3��Na2CO3��Na2CO3��NaHCO3��NaHCO3��������뷴Ӧ�����Һ�У�HCl�Ⱥ�NaOH��Ӧ���ٺ�Na2CO3��Ӧ��CO32-+H+=HCO3-������ٷ���HCO3-+H+=H2O+CO2����
II�������������ᡢ�������Ʒ�Ӧ�ķ���ʽ���
III������ԭ���غ���
I����1����A����£�����100mL��HCl�����ʵ���Ϊ0.1L��0.2 mol��L��1=0.02mol�������ʼ������ų����ټ���50mL���ᣨHCl�����ʵ���Ϊ0.05L��0.2 mol��L��1=0.01mol������ﵽ��࣬����������NaOH��Na2CO3����������ķ���ʽ��0.01molHCl����0.01mol NaHCO3
������0.01mol Na2CO3��0.01molNa2CO3����0.01molHCl�������0.01molHCl�Ǻ�NaOH������Ӧ���ĵģ�����NaOH�����ʵ���Ҳ��0.01mol������NaOH��Na2CO3���ʵ���֮��Ϊ1:1��
��2������150mL����ʱ����Һ�е�����ֻ��NaCl������ԭ���غ㣬��ȡ����10mL��Ӧ�����Һ�У�n(NaOH)=n(HCl)=0.15L��0.2 mol��L��1=0.03mol������ԭ����������Һ�����ʵ���Ũ��Ϊ![]() =3mol/L��
=3mol/L��
II���������ᡢ����������Һ��Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��2Al+6HCl=2AlCl3+3H2����2Al+2NaOH+2H2O=2NaAlO2+3H2�������������������Һ����������ʵ���Ũ�ȶ���ȣ������ʵ����ʵ�����ȣ���HCl��NaOH��Ϊ6mol��������������ֱ�Ϊ3mol��9mol��ͬ��ͬѹ�����ʵ���֮�ȵ�������ȣ����Բ���H2�����֮����3:9=1:3��
III����һ������Fe��FeO��Fe2O3�Ļ�����м���100 mLŨ��Ϊ2.0 mol��L��1�����ᣨ��HCl�����ʵ���Ϊ0.1L�� 2.0 mol��L��1=0.2mol����ǡ��ʹ�������ȫ�ܽ⣬������Һ�м���KSCN��Һ����Ѫ��ɫ���֣�˵����Һ�е�����ֻ��FeCl2��������Ԫ���غ㣬n(FeCl2)=![]() n(Cl-)=0.1mol������������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ܵõ��������ʵ�����FeCl2�����ʵ�����ȣ���0.1mol����������������0.1mol��56g/mol=5.6g������������ԭ���غ㣨�������غ㣩��
n(Cl-)=0.1mol������������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ܵõ��������ʵ�����FeCl2�����ʵ�����ȣ���0.1mol����������������0.1mol��56g/mol=5.6g������������ԭ���غ㣨�������غ㣩��

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�����Ŀ��ij��ҵ�����У����뷴Ӧ���Ļ��������NO��O2�����ʵ��������քeΪ0.10��0.06��������ѧ��Ӧ2NO(g)+O2(g) ![]() 2NO2(g)��������������ͬʱ�����ʵ���������±���
2NO2(g)��������������ͬʱ�����ʵ���������±���
ѹǿ/����105Pa�� | �¶�/�� | NO�ﵽ����ת������Ҫʱ��/s | ||
50% | 90% | 98% | ||
1.0 | 30 | 12 | 250 | 2830 |
90 | 25 | 510 | 5760 | |
8.0 | 30 | 0.2 | 3.9 | 36 |
90 | 0.6 | 7.9 | 74 | |
���ݱ������ݣ�����˵����ȷ����
A.�����¶ȣ���Ӧ���ʼӿ�
B.����ѹǿ����Ӧ���ʱ���
C.��1.0��105 Pa��90�������£���ת����Ϊ98%ʱ��Ӧ�Ѵ�ƽ��
D.�����뷴Ӧ���Ļ������Ϊa mol����Ӧ������![]() ��ʾ������8.0��105Pa��30�������£�ת���ʴ�50%����90%ʱ��NO�ķ�Ӧ����Ϊ
��ʾ������8.0��105Pa��30�������£�ת���ʴ�50%����90%ʱ��NO�ķ�Ӧ����Ϊ![]() mols-1
mols-1