��Ŀ����
7�����к�NaCl��Na2SO4��Na2CO3��NaHCO3�Ĺ������ijͬѧΪ�ⶨ���и���ֵĺ�����ȡ������Ʒ����ˮ�������������ʵ�����̣������Լ���Ϊ��������Ӧ�١��۾�Ϊ���ֽⷴӦ����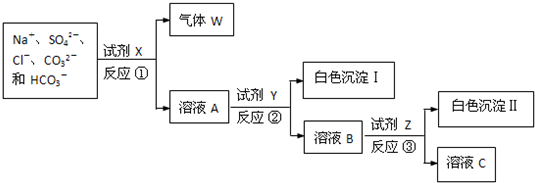
��ش�
��1������W�Ļ�ѧʽ��CO2��
��2����Ӧ�ٵ����ӷ���ʽ��CO32-+2H+�TCO2��+H2O��HCO3-+H+�TCO2��+H2O��
��3���Լ�X��Y��Z������������Һ�����ᱵ��Һ����������Һ��
��4������˵����ȷ����ab������ĸ����
a����ҺA����ɫ��ӦΪ��ɫ
b����Ӧ�ں͢�֮�������й��˲���
c�����������W���������״��������ɫ������͢���������ɼ��������ֵ�����
���� NaCl��Na2SO4��Na2CO3��NaHCO3�Ĺ��������м���ϡ���ᣬ��Ӧ���ɵ�����Ϊ������̼���壬��WΪ������̼����ҺA�к���Na+��SO42-��Cl-�ͼ�������ᣬ�ʻ�����H+��NO3-����������Һ����õķ����ǹ��ˣ������Լ�Y�����ᱵ�����ȼ���AgNO3�����ͬʱ����Ag2SO4��AgCl��������Ӧ�ȼ��������BaCl2[��Ba��NO3��2]������BaSO4������Ȼ������Һ�м��������AgNO3��ʹCl-ȫ��ת��ΪAgCl��������������Һ�м��������Na2CO3��ʹ��Һ�е�Ag+��Ba2+��ȫ���������������ҺΪNaNO3��Na2CO3�Ļ�������ϡHNO3�����������������ɵù���NaNO3���ݴ˽�ɣ�
��� �⣺NaCl��Na2SO4��Na2CO3��NaHCO3�Ĺ��������м���ϡ���ᣬ��Ӧ���ɵ�����Ϊ������̼���壬��WΪ������̼����ҺA�к���Na+��SO42-��Cl-�ͼ�������ᣬ�ʻ�����H+��NO3-����������Һ����õķ����ǹ��ˣ������Լ�Y�����ᱵ�����ȼ���AgNO3�����ͬʱ����Ag2SO4��AgCl��������Ӧ�ȼ��������BaCl2[��Ba��NO3��2]������BaSO4������Ȼ������Һ�м��������AgNO3��ʹCl-ȫ��ת��ΪAgCl��������������Һ�м��������Na2CO3��ʹ��Һ�е�Ag+��Ba2+��ȫ���������������ҺΪNaNO3��Na2CO3�Ļ�������ϡHNO3�����������������ɵù���NaNO3��
��1�����ݷ�����֪������W��CO2���ʴ�Ϊ��CO2��
��2��̼����������ᷴӦ��CO32-+2H+�TCO2��+H2O��HCO3-�������ᷴӦ��HCO3-+H+�TCO2��+H2O���ʴ�Ϊ��CO32-+2H+�TCO2��+H2O��HCO3-+H+�TCO2��+H2O��
��3�����ݷ�����֪��X��ϡ���ᣬY��Ba��NO3��2��Z����������Һ���ʴ�Ϊ��������Һ�����ᱵ��Һ����������Һ��
��4��a����ҺA������Ԫ�أ���ɫ��ӦΪ��ɫ��
b���ں͢�֮�������й��˲���������������Һ�ֿ���
c���ⶨ����ֵĺ�������Ҫ��������У����������������ɫ������͢�ֱ������������W���������״������������W������������ȱ�ٻ������������
�ʴ�Ϊ��ab��
���� ������Ҫ������ǻ����ļ�������룬�漰���ֽⷴӦ���Լ���ѡ��ȣ��ѶȲ����ݳ����Dz��������µ����ʡ�����Ӱ�챻�ᴿ�����ʵ����ʺ��������Ҳ������У�����ʱҪ������ʵ��������ʺͻ�ѧ���ʽ��з��룮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �Ҵ���CH3-O-CH3 | B�� | CH3COOH���������� | ||
| C�� | O2��O3 | D�� | ���ۺ���ά�� |
| A�� | N2��g��+3H2��g��?2NH3��g�� | B�� | H2��g��+I2��g��?2HI��g�� | ||
| C�� | C��s��+H2O��g��?CO��g��+H2��g�� | D�� | CO2��g��+2NH3��g��?CO��NH2��2��s��+H2O��g�� |
| A�� | �ۢ٢ܢڢ� | B�� | �٢ۢܢڢ� | C�� | �ۢڢ٢ܢ� | D�� | �ݢڢܢ٢� |
| A�� | ������п�����·���������� | |
| B�� | п���������������Ǹ��� | |
| C�� | ����ʱ����������ҺpH��С��������pH���� | |
| D�� | ��Һ��OH-�������ƶ���K+��H+���ƶ� |
| A�� | ����ˮʱ����Һ��c��H+�� ��c��OH-�� ����С | |
| B�� | ��������NaOH���壬ƽ��������Ӧ�����ƶ� | |
| C�� | ��������0.1mol/L HCl��Һ����Һ��c��H+����С | |
| D�� | ��������CH3COONa���壬ƽ��������Ӧ�����ƶ� |



