��Ŀ����
13������1molKAl��SO4��2����Һ����μ�������������Һ����ַ�Ӧ������˵������ȷ���ǣ�������| A�� | ��Al3+ǡ����ȫ����ʱ��������������1.5mol | |
| B�� | ��SO42-ǡ����ȫ����ʱ��Al3+ȫ��ת��ΪAl��OH��3 | |
| C�� | ������Һ�м���1.5mol��������ʱ����Ӧ�����������ӷ���ʽ��ʾ��2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� | |
| D�� | ��Ӧ�������������������������ʵ��������������������������� |
���� 1molKAl��SO4��2����μ���Ba��OH��2��Һ����Ӧ���̿ɷ������Σ�2KAl��SO4��2+3Ba��OH��2=K2SO4+2Al��OH��3��+3BaSO4���٣�KAl��SO4��2+2Ba��OH��2=KAlO2+2BaSO4��+2H2O�ڣ�
��һ�Σ��ӿ�ʼ����Ba��OH��2��1.5molʱ����Ӧ���ٽ��У���Һ�е�Al3+��Ba2+��OH-���Ӿ�ת���ɳ�����
�ڶ��Σ�������Ba��OH��2��1.5mol��2mol֮��ʱ��Ba2+������SO42-��Ӧ����BaSO4��ͬʱ�ٷ�Ӧ���ɵ�Al��OH��3��OH-��Ӧ����AlO2-��
�����Σ������뵽Ba��OH��22molʱ�����ڽ��У����ɵ�Al��OH��3ȫ��ת��ΪAlO2-��������2molBaSO4��
��� �⣺A����һ�Σ��ӿ�ʼ����Ba��OH��2��1.5molʱ����Ӧ��2KAl��SO4��2+3Ba��OH��2=K2SO4+2Al��OH��3��+3BaSO4���ٽ��У�����1molAl3+ǡ����ȫ����ʱ��������������1.5mol����A��ȷ��
B��1mol KAl��SO4��2����Һ�к���2mol��SO42-����SO42-ǡ����ȫ����ʱ����Ҫ2mol��Ba2+����������2mol��Ba��OH��2����KAl��SO4��2+2Ba��OH��2=KAlO2+2BaSO4��+2H2O��Al3+ȫ��ת��Ϊƫ��������ӣ���B����
C��������Һ�м���1.5mol��������ʱ��������Ӧ��2KAl��SO4��2+3Ba��OH��2=K2SO4+2Al��OH��3��+3BaSO4�������ӷ���ʽ��ʾΪ��2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4������C��ȷ��
D���������������������ʵ�����������ʼ���������࣬��������������ܽ⣬�ʳ��������ʵ�����������С����D����
��ѡBD��
���� ������һ����������֮��ķ�Ӧ֪ʶ����Ŀ������ѧ�������ͽ����������������������仯����������ǽ��ؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ������Ϊ17��������Ϊ20����ԭ�ӣ�${\;}_{17}^{20}Cl$ | |
| B�� | �����ʵ�����ˮ����ˮ������ͬ��Ŀ�������� | |
| C�� | �ȷ��ӵĵ���ʽ�� | |
| D�� | �����ʵ������������ֱ�����������������������Һ��ȫ��Ӧʱת�Ƶĵ�������ͬ |
| A�� | $\frac{B}{A}$ mol•L-1 | B�� | $\frac{2A}{B}$mol•L-1 | C�� | $\frac{A}{2B}$mol•L-1 | D�� | $\frac{B}{2A}$mol•L-1 |
| A�� | ��1L2mol•L-1��NH4Cl��Һ�У�ȡ�����е�500ml���������ʵ���Ũ��Ϊ1mol•L-1 | |
| B�� | 1mol•L-1��NaCl��Һ�������ÿ��NaCl��Һ�к���1molNaCl | |
| C�� | ��4gNaOH�ܽ���100mlˮ�У����γɵ���Һ�����ʵ���Ũ��1mol•L-1 | |
| D�� | ��50m2mol•L-1��CaCl2��Һ��ˮϡ�ͳ�100ml����ϡ�ͺ����Һ��������CaCl2�����ʵ������ԭ����һ�� |
| A�� | ���߾��������� | |
| B�� | ���߾����ڻ���� | |
| C�� | ǰ�������л��߷��ӻ������������������� | |
| D�� | ǰ�������л��߷��ӻ�������������л������ |
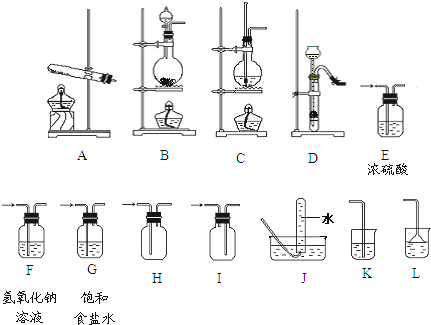
����д���б����еĿհ״���
| ��� | ���� | װ�õ�����˳���÷��ű�ʾ�� | ��Ҫ�����Լ� ���� | ʵ������ȡ������ �ķ�Ӧ����ʽ �������ӷ�Ӧ��д���ӷ�Ӧ����ʽ�� |
| ��1�� | CO2 | �Ʊ����ռ� | ||
| ��2�� | NH3 | �Ʊ����ռ� ��β������ | �����������ѡ�õ��Լ� ��ʯ�� | |
| ��3�� | Cl2 | �Ʊ���������������ռ���β������ | ����β�� ���Լ� ����������Һ | |
| ��4�� | �Ʊ����������ռ� C��F��J |
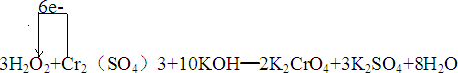 ��
�� 